Difference between revisions of "Template:Article of the week"
Shawndouglas (talk | contribs) (Updated article of the week text) |
Shawndouglas (talk | contribs) (Updated article of the week text) |
||
| Line 1: | Line 1: | ||
<div style="float: left; margin: 0.5em 0.9em 0.4em 0em;">[[File: | <div style="float: left; margin: 0.5em 0.9em 0.4em 0em;">[[File:Fig7 Maury FrontDigHlth2021 3.jpg|240px]]</div> | ||
'''"[[Journal: | '''"[[Journal:An automated dashboard to improve laboratory COVID-19 diagnostics management|An automated dashboard to improve laboratory COVID-19 diagnostics management]]"''' | ||
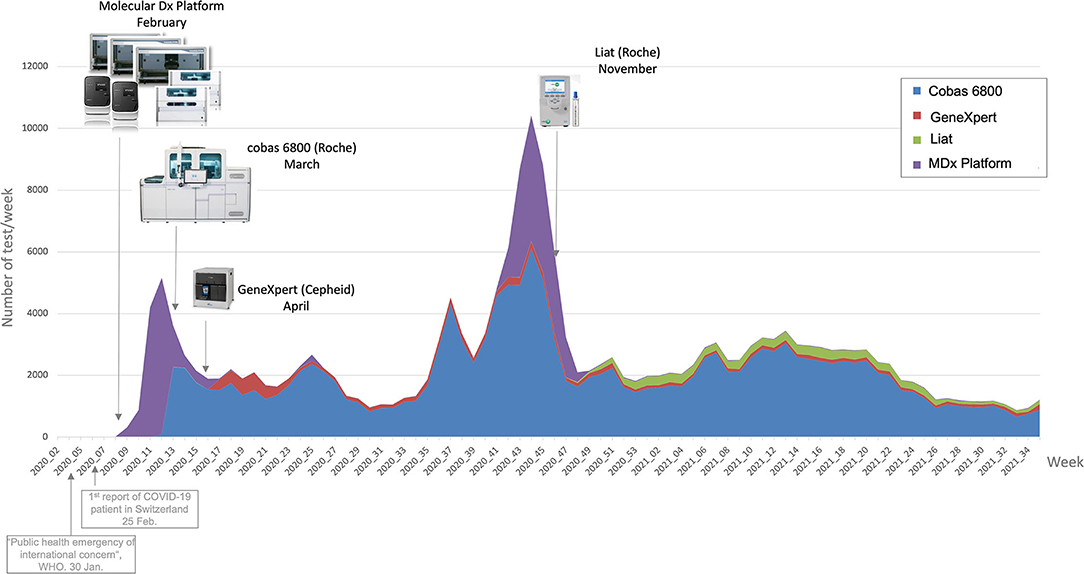
[[ | In response to the [[COVID-19]] [[pandemic]], our microbial diagnostic [[laboratory]] located in a university [[hospital]] has implemented several distinct [[SARS-CoV-2]] [[reverse transcription polymerase chain reaction]] (RT-PCR) systems in a very short time. More than 148,000 tests have been performed over 12 months, which represents about 405 tests per day, with peaks to more than 1,500 tests per days during the second wave. This was only possible thanks to [[Laboratory automation|automation]] and digitalization, to allow high-throughput, acceptable time to results and to maintain test reliability. An automated dashboard was developed to give access to key performance indicators (KPIs) to improve laboratory operational management. RT-PCR data extraction of four respiratory viruses—SARS-CoV-2, influenza A and B, and RSV—from our [[laboratory information system]] (LIS) was automated. This included ... ('''[[Journal:An automated dashboard to improve laboratory COVID-19 diagnostics management|Full article...]]''')<br /> | ||
<br /> | <br /> | ||
''Recently featured'': | ''Recently featured'': | ||
{{flowlist | | {{flowlist | | ||
* [[Journal:Management of post-analytical processes in the clinical laboratory according to ISO 15189:2012: Considerations about the management of clinical samples, ensuring quality of post-analytical processes and laboratory information management|Management of post-analytical processes in the clinical laboratory according to ISO 15189:2012: Considerations about the management of clinical samples, ensuring quality of post-analytical processes and laboratory information management]] | |||
* [[Journal:A survival guide for the rapid transition to a fully digital workflow: The Caltagirone example|A survival guide for the rapid transition to a fully digital workflow: The Caltagirone example]] | * [[Journal:A survival guide for the rapid transition to a fully digital workflow: The Caltagirone example|A survival guide for the rapid transition to a fully digital workflow: The Caltagirone example]] | ||
* [[Journal:From biobank and data silos into a data commons: Convergence to support translational medicine|From biobank and data silos into a data commons: Convergence to support translational medicine]] | * [[Journal:From biobank and data silos into a data commons: Convergence to support translational medicine|From biobank and data silos into a data commons: Convergence to support translational medicine]] | ||
}} | }} | ||
Revision as of 15:20, 19 September 2022
"An automated dashboard to improve laboratory COVID-19 diagnostics management"
In response to the COVID-19 pandemic, our microbial diagnostic laboratory located in a university hospital has implemented several distinct SARS-CoV-2 reverse transcription polymerase chain reaction (RT-PCR) systems in a very short time. More than 148,000 tests have been performed over 12 months, which represents about 405 tests per day, with peaks to more than 1,500 tests per days during the second wave. This was only possible thanks to automation and digitalization, to allow high-throughput, acceptable time to results and to maintain test reliability. An automated dashboard was developed to give access to key performance indicators (KPIs) to improve laboratory operational management. RT-PCR data extraction of four respiratory viruses—SARS-CoV-2, influenza A and B, and RSV—from our laboratory information system (LIS) was automated. This included ... (Full article...)
Recently featured:
- Management of post-analytical processes in the clinical laboratory according to ISO 15189:2012: Considerations about the management of clinical samples, ensuring quality of post-analytical processes and laboratory information management
- A survival guide for the rapid transition to a fully digital workflow: The Caltagirone example
- From biobank and data silos into a data commons: Convergence to support translational medicine