Journal:Comprehensive improvements in the emergency laboratory test process based on information technology
| Full article title | Comprehensive improvements in the emergency laboratory test process based on information technology |
|---|---|
| Journal | BMC Medical Informatics and Decision Making |
| Author(s) | Zhang, Liang; Liu, Zhen Hua; Lv, Yin Jiang; Fu, Shui; Luo, Zhang Mei; Guo, Mei Li |
| Author affiliation(s) | Zhejiang University School of Medicine, People’s Hospital of Cangnan Zhejiang |
| Primary contact | guomeili2000 at 163 dot com |
| Year published | 2023 |
| Volume and issue | 23 |
| Article # | 292 |
| DOI | 10.1186/s12911-023-02387-x |
| Distribution license | Creative Commons Attribution 4.0 International |
| Website | https://bmcmedinformdecismak.biomedcentral.com/articles/10.1186/s12911-023-02387-x |
| Download | https://bmcmedinformdecismak.biomedcentral.com/counter/pdf/10.1186/s12911-023-02387-x.pdf (PDF) |
|
|
This article should be considered a work in progress and incomplete. Consider this article incomplete until this notice is removed. |
Abstract
Objective: To explore the application effects of information technology (IT) on emergency laboratory testing procedures.
Methods: In this study, IT-based optimization of the emergency laboratory testing process was implemented between October and December 2021. Emergency laboratory test reports from January to September 2021 were placed into the pre-optimized group, while those from January to September 2022 were categorized into the post-optimized group. Additionally, the emergency laboratory test report time, emergency laboratory test report time limit coincidence rate, error rate, and employee and patient satisfaction levels in individual months and across the whole period were described. Moreover, changes in the above indicators before and after the implementation of IT-based optimization were explored, and the application effects of IT-based optimization were also evaluated.
Results: The emergency laboratory test report times after the implementation of IT-based optimization were shorter than those before IT-based optimization (P < 0.05). The total number of laboratory test items before and after information optimization amounted to 222,139 and 259,651, respectively. Also, IT-based optimization led to an increase in the emergency laboratory test report time limit coincidence rate from 98.77% to 99.03% (P < 0.05), while the emergency laboratory test report error rate fell from 0.77‱ to 0.15‱ (P < 0.05). Additionally, IT-based optimization resulted in increases in both employee satisfaction, from 80.65% to 93.55% (N = 31, P > 0.05), and patient satisfaction, from 93.06% to 98.44% (P < 0.05).
Conclusion: The automation and IT-based optimization of the emergency laboratory testing process significantly reduces the emergency laboratory test report time and error rate. Additionally, IT-driven optimization enhances the alignment of emergency laboratory test report deadlines and enhances the overall quality and safety of emergency laboratory testing.
Keywords: emergency laboratory test, information technology, process optimization, satisfaction, turnaround time
Introduction
With the progress of medical reforms in China, there is now an increasing demand for high-quality healthcare services. Stricter requirements regarding the quality and efficiency of laboratory tests have also been proposed in clinical practice. On that basis, to further improve the quality and speed of laboratory test reports, clinicians are exploring methods to satisfy the demands of clinical applications and patients for the accurate and timely processing of laboratory tests. In China, most patients with acute onset, severe symptoms, or complex conditions are initially admitted to the emergency department, which increases the aggregation of patients with acute and critical conditions. [1] In contrast to conventional patients, patients in the emergency department often simultaneously require emergency rescue and disease diagnosis. Therefore, the emergency department must provide the ideal allocation of limited resources in the shortest possible time. Additionally, there are more stringent requirements for emergency laboratory test results, since delayed or inaccurate test results may affect the clinical diagnosis and lead to delayed or even inappropriate treatment. [2, 3]
Emergency laboratory tests are a vital component of emergency medical treatment. A comprehensive array of emergency laboratory test items and accurate and timely test reports provide adequate support for emergency patients to receive effective care within the optimal rescue window. [4, 5] Moreover, the rapid evaluation of patients can be achieved within the emergency department in case of sudden onset of disease, trauma, or public health emergencies. Meanwhile, the emergency department should cooperate with various hospital departments to make clinical decisions swiftly, thereby saving the lives of patients and avoiding further aggravation of disease. As the front line of emergency diagnosis and treatment, emergency laboratory tests play a crucial role in the treatment of patients with acute, severe, and critical conditions. Moreover, emergency laboratory test results exert a significant impact on making timely and accurate clinical decisions. [6] Therefore, reducing emergency laboratory test report times is essential for enhancing emergency treatment capability.
According to the accreditation criteria for ISO 15189 Medical laboratories: Requirements for quality and competence [7], turnaround time (TAT) is defined as “the duration between two designated points in the process before, during, and after laboratory tests.” TAT is employed to reflect the working efficiency of laboratories and is the preferred indicator for evaluating laboratory service quality. The laboratory test report time refers to the laboratory TAT, namely the duration (in minutes) from the time when the laboratory receives the specimen to the time when the report is sent. As a result, TAT has become an important controllable quality indicator for laboratory testing. There are specific regulations and requirements regarding this indicator in China's 2011 Detailed Implementation Rules for the Accreditation Standards of Tertiary General Hospitals (三级综合医院评审标准实施细则). Additionally, TAT was included as one of the 15 quality indicators issued by the National Centre for Clinical Laboratories in 2015, and it has been verified as an important quality indicator in laboratory tests. As specified in ISO 15189 Medical laboratories: Requirements for quality and competence [8], laboratory quality management includes the management of test result authenticity and reliability and also covers the management of various factors that may affect test results. The laboratory test report time is a key factor that affects the diagnosis and treatment of patients. In numerous clinical laboratories worldwide, the laboratory test report time is regarded as an important observation indicator for continuous quality improvement, while limitations in the emergency laboratory test report time have been highlighted. [9,10,11]
The application range of laboratory information technology has become an important symbol of scientific laboratory quality management. By applying information management technology to analyze and monitor the time nodes of key links in laboratory tests, the main test line, namely the “laboratory test report,” can be more effectively managed. Although IT has been applied to laboratory management and has achieved favorable results [12,13,14], transformation and optimization based on this technology are performed differently according to the procedures of individual hospitals. Hence, it is necessary to incorporate IT into the overall emergency laboratory test process and constantly conduct summarizations and optimizations in clinical practice, thereby maximizing automation and early warning during each step. Based on this, the working efficiency can be improved and the laboratory test report time can be reduced, which is consistent with the core values of the hospital: “Focus on the perceptions of patients and employees.”
This scientific study lays a solid foundation for appropriately applying emergency laboratory tests in clinical practice, enhancing the quality of medical services, ensuring patient safety, and improving patient satisfaction.
Data and methods
General data
As a contemporary tertiary second-class general hospital, our institution seamlessly integrates medical treatment, scientific research, teaching, rehabilitation, prevention, and healthcare services. We cater to approximately 1.8 million outpatients and admit around 50,000 inpatients annually. The hospital encompasses a sprawling site area of 108 mu, with a combined utilization area totaling 140,000 square meters. Our facilities include 32 open wards, housing a total of 1,000 beds, and a dedicated workforce of over 1,500 employees. In our department, the IT-based optimization of the emergency laboratory test process was implemented between October and December 2021. The emergency laboratory test reports from January to September 2021 were assigned to the pre-optimization group, while those from January to September 2022 were categorized into the post-optimization group. The emergency laboratory test report time limit for routine blood test + CRP, routine urine test, routine fecal test, blood group test, urine pregnancy test, and blood gas analysis was ≤ 30 minutes, while that of biochemical, immune, and other tests was ≤ 1 hour.
Methods
Materials
A laboratory information management system (LIMS) was purchased from Hangzhou Lingyun Technology Co. Any problems encountered in emergency laboratory tests were discussed within the department and then reported to software engineers. Subsequently, IT engineers conducted repairs and optimizations to solve these issues.
Circumstances and problems in the laboratory test process before optimization
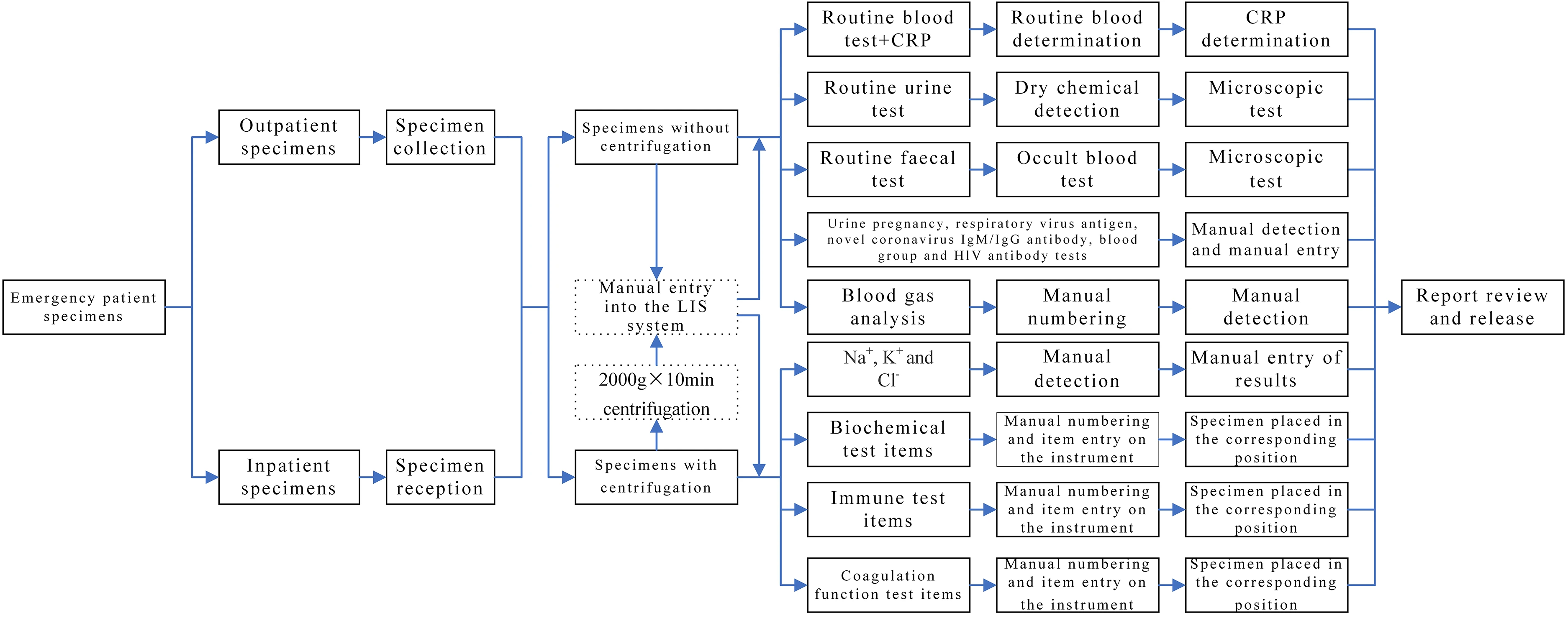
Specimens obtained from patients in outpatient or emergency departments labeled as “emergency” and collected by clinicians in clinical departments for laboratory tests are referred to as non-hospitalized specimens. In contrast, specimens collected from inpatients with “emergency” labels in clinical departments for laboratory tests are designated as hospitalized specimens. The emergency laboratory test specimens of outpatients were manually entered into the corresponding category in the LIMS system, with a category specified for each item. However, for inpatients, the emergency laboratory test specimens were first input into the receiving system and then manually entered into the corresponding category in the LIMS system. Subsequently, an automated or manual test was conducted. The results were then submitted and reports reviewed as necessary, while all problems were resolved according to the operating procedures. The specific laboratory test items are listed in Table 1, and the detailed workflow is presented in Fig. 1.
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
References
Notes
This presentation is faithful to the original, with only a few minor changes to presentation. In some cases important information was missing from the references, and that information was added.