Journal:Comprehensive improvements in the emergency laboratory test process based on information technology
| Full article title | Comprehensive improvements in the emergency laboratory test process based on information technology |
|---|---|
| Journal | BMC Medical Informatics and Decision Making |
| Author(s) | Zhang, Liang; Liu, Zhen Hua; Lv, Yin Jiang; Fu, Shui; Luo, Zhang Mei; Guo, Mei Li |
| Author affiliation(s) | Zhejiang University School of Medicine, People’s Hospital of Cangnan Zhejiang |
| Primary contact | guomeili2000 at 163 dot com |
| Year published | 2023 |
| Volume and issue | 23 |
| Article # | 292 |
| DOI | 10.1186/s12911-023-02387-x |
| Distribution license | Creative Commons Attribution 4.0 International |
| Website | https://bmcmedinformdecismak.biomedcentral.com/articles/10.1186/s12911-023-02387-x |
| Download | https://bmcmedinformdecismak.biomedcentral.com/counter/pdf/10.1186/s12911-023-02387-x.pdf (PDF) |
Abstract
Objective: To explore the application effects of information technology (IT) on emergency laboratory testing procedures.
Methods: In this study, IT-based optimization of the emergency laboratory testing process was implemented between October and December 2021. Emergency laboratory test reports from January to September 2021 were placed into the pre-optimized group, while those from January to September 2022 were categorized into the post-optimized group. Additionally, the emergency laboratory test report time, emergency laboratory test report time limit coincidence rate, error rate, and employee and patient satisfaction levels in individual months and across the whole period were described. Moreover, changes in the above indicators before and after the implementation of IT-based optimization were explored, and the application effects of IT-based optimization were also evaluated.
Results: The emergency laboratory test report times after the implementation of IT-based optimization were shorter than those before IT-based optimization (P < 0.05). The total number of laboratory test items before and after information optimization amounted to 222,139 and 259,651, respectively. Also, IT-based optimization led to an increase in the emergency laboratory test report time limit coincidence rate from 98.77% to 99.03% (P < 0.05), while the emergency laboratory test report error rate fell from 0.77‱ to 0.15‱ (P < 0.05). Additionally, IT-based optimization resulted in increases in both employee satisfaction, from 80.65% to 93.55% (N = 31, P > 0.05), and patient satisfaction, from 93.06% to 98.44% (P < 0.05).
Conclusion: The automation and IT-based optimization of the emergency laboratory testing process significantly reduces the emergency laboratory test report time and error rate. Additionally, IT-driven optimization enhances the alignment of emergency laboratory test report deadlines and enhances the overall quality and safety of emergency laboratory testing.
Keywords: emergency laboratory test, information technology, process optimization, satisfaction, turnaround time
Introduction
With the progress of medical reforms in China, there is now an increasing demand for high-quality healthcare services. Stricter requirements regarding the quality and efficiency of laboratory tests have also been proposed in clinical practice. On that basis, to further improve the quality and speed of laboratory test reports, clinicians are exploring methods to satisfy the demands of clinical applications and patients for the accurate and timely processing of laboratory tests. In China, most patients with acute onset, severe symptoms, or complex conditions are initially admitted to the emergency department, which increases the aggregation of patients with acute and critical conditions.[1] In contrast to conventional patients, patients in the emergency department often simultaneously require emergency rescue and disease diagnosis. Therefore, the emergency department must provide the ideal allocation of limited resources in the shortest possible time. Additionally, there are more stringent requirements for emergency laboratory test results, since delayed or inaccurate test results may affect the clinical diagnosis and lead to delayed or even inappropriate treatment.[2][3]
Emergency laboratory tests are a vital component of emergency medical treatment. A comprehensive array of emergency laboratory test items and accurate and timely test reports provide adequate support for emergency patients to receive effective care within the optimal rescue window.[4][5] Moreover, the rapid evaluation of patients can be achieved within the emergency department in case of sudden onset of disease, trauma, or public health emergencies. Meanwhile, the emergency department should cooperate with various hospital departments to make clinical decisions swiftly, thereby saving the lives of patients and avoiding further aggravation of disease. As the front line of emergency diagnosis and treatment, emergency laboratory tests play a crucial role in the treatment of patients with acute, severe, and critical conditions. Moreover, emergency laboratory test results exert a significant impact on making timely and accurate clinical decisions.[6] Therefore, reducing emergency laboratory test report times is essential for enhancing emergency treatment capability.
According to the accreditation criteria for ISO 15189 Medical laboratories: Requirements for quality and competence[7], turnaround time (TAT) is defined as “the duration between two designated points in the process before, during, and after laboratory tests.” TAT is employed to reflect the working efficiency of laboratories and is the preferred indicator for evaluating laboratory service quality. The laboratory test report time refers to the laboratory TAT, namely the duration (in minutes) from the time when the laboratory receives the specimen to the time when the report is sent. As a result, TAT has become an important controllable quality indicator for laboratory testing. There are specific regulations and requirements regarding this indicator in China's 2011 Detailed Implementation Rules for the Accreditation Standards of Tertiary General Hospitals (三级综合医院评审标准实施细则). Additionally, TAT was included as one of the 15 quality indicators issued by the National Centre for Clinical Laboratories in 2015, and it has been verified as an important quality indicator in laboratory tests. As specified in ISO 15189 Medical laboratories: Requirements for quality and competence[8], laboratory quality management includes the management of test result authenticity and reliability and also covers the management of various factors that may affect test results. The laboratory test report time is a key factor that affects the diagnosis and treatment of patients. In numerous clinical laboratories worldwide, the laboratory test report time is regarded as an important observation indicator for continuous quality improvement, while limitations in the emergency laboratory test report time have been highlighted.[9][10][11]
The application range of laboratory information technology has become an important symbol of scientific laboratory quality management. By applying information management technology to analyze and monitor the time nodes of key links in laboratory tests, the main test line, namely the “laboratory test report,” can be more effectively managed. Although IT has been applied to laboratory management and has achieved favorable results[12][13][14], transformation and optimization based on this technology are performed differently according to the procedures of individual hospitals. Hence, it is necessary to incorporate IT into the overall emergency laboratory test process and constantly conduct summarizations and optimizations in clinical practice, thereby maximizing automation and early warning during each step. Based on this, the working efficiency can be improved and the laboratory test report time can be reduced, which is consistent with the core values of the hospital: “Focus on the perceptions of patients and employees.”
This scientific study lays a solid foundation for appropriately applying emergency laboratory tests in clinical practice, enhancing the quality of medical services, ensuring patient safety, and improving patient satisfaction.
Data and methods
General data
As a contemporary tertiary second-class general hospital, our institution seamlessly integrates medical treatment, scientific research, teaching, rehabilitation, prevention, and healthcare services. We cater to approximately 1.8 million outpatients and admit around 50,000 inpatients annually. The hospital encompasses a sprawling site area of 108 mu, with a combined utilization area totaling 140,000 square meters. Our facilities include 32 open wards, housing a total of 1,000 beds, and a dedicated workforce of over 1,500 employees. In our department, the IT-based optimization of the emergency laboratory test process was implemented between October and December 2021. The emergency laboratory test reports from January to September 2021 were assigned to the pre-optimization group, while those from January to September 2022 were categorized into the post-optimization group. The emergency laboratory test report time limit for routine blood test + CRP, routine urine test, routine fecal test, blood group test, urine pregnancy test, and blood gas analysis was ≤ 30 minutes, while that of biochemical, immune, and other tests was ≤ 1 hour.
Methods
Materials
A laboratory information management system (LIMS) was purchased from Hangzhou Lingyun Technology Co. Any problems encountered in emergency laboratory tests were discussed within the department and then reported to software engineers. Subsequently, IT engineers conducted repairs and optimizations to solve these issues.
Circumstances and problems in the laboratory test process before optimization
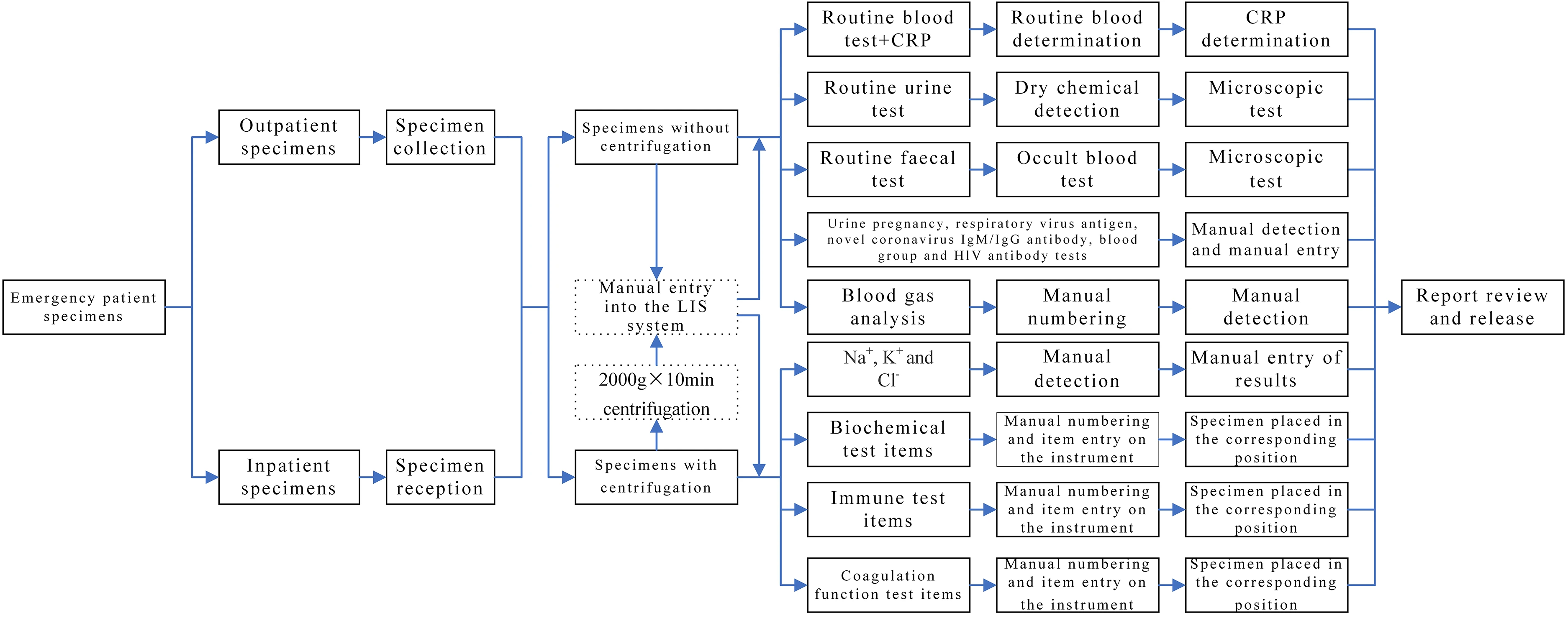
Specimens obtained from patients in outpatient or emergency departments labeled as “emergency” and collected by clinicians in clinical departments for laboratory tests are referred to as non-hospitalized specimens. In contrast, specimens collected from inpatients with “emergency” labels in clinical departments for laboratory tests are designated as hospitalized specimens. The emergency laboratory test specimens of outpatients were manually entered into the corresponding category in the LIMS system, with a category specified for each item. However, for inpatients, the emergency laboratory test specimens were first input into the receiving system and then manually entered into the corresponding category in the LIMS system. Subsequently, an automated or manual test was conducted. The results were then submitted and reports reviewed as necessary, while all problems were resolved according to the operating procedures. The specific laboratory test items are listed in Table 1, and the detailed workflow is presented in Fig. 1.
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Existing problems
(1) The instruments were outdated since most of them were purchased during the relocation of our hospital in 2013. Since a majority of the instruments were not equipped with two-way communication, it was necessary to enter the test items and record the test results manually in some instruments. (2) The specimen’s name could only be entered by manual code scanning, which amplified the workload and increased the likelihood of missing entries or incorrect set entries. (3) There were no relevant prompts throughout the whole process and no relevant tracking function for adverse events such as sample omission or timeout. (4) The items with critical values were reported by manual telephone notification or manual registration, which was time-consuming and also led to data entry errors (Table 2).
| ||||||||||||||||||||||||
Identification of problems in clinical practice and relevant optimizations
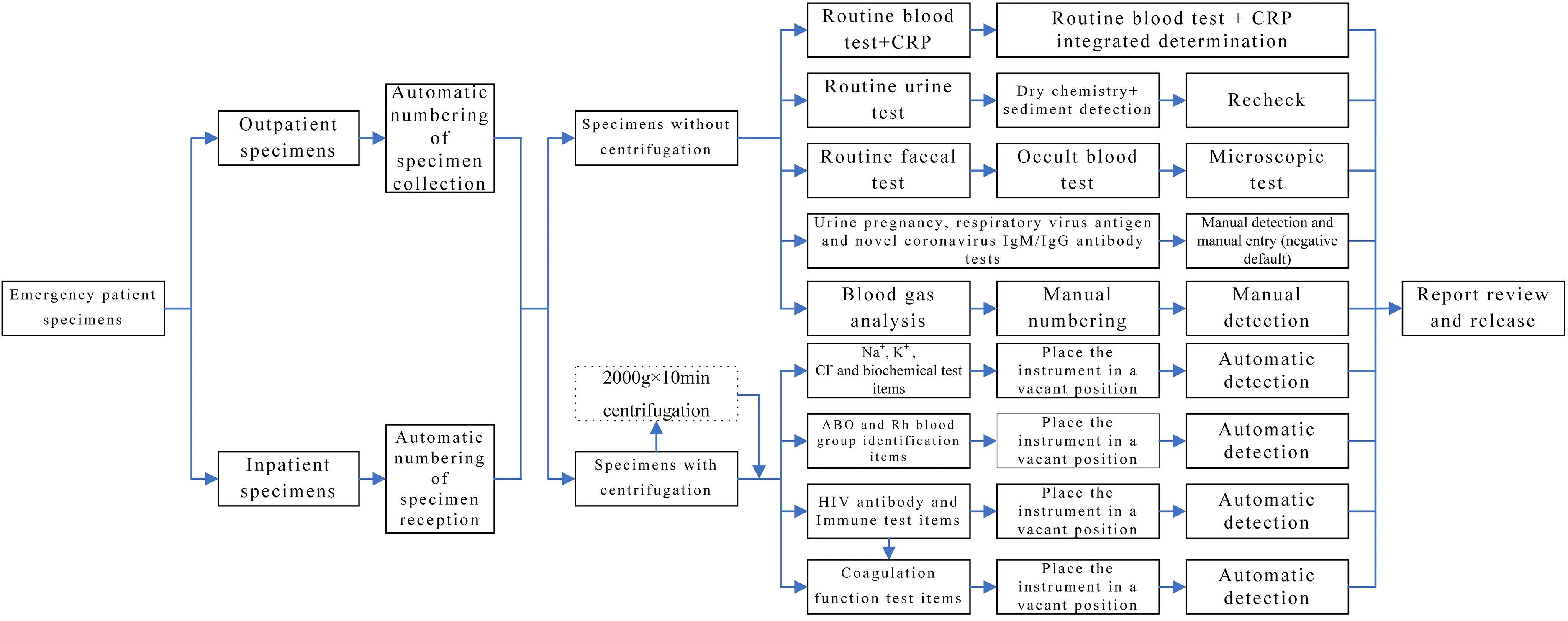
(1) Update equipment: The problems associated with instrument operation were dealt with. Some instruments were equipped with two-way communication with the aid of the manufacturer and the LIMS system engineer. Additionally, some new instruments were purchased to replace the existing ones, and all new instruments were equipped with two-way communication during installation. After automation and information integration of the equipment across the whole process was realized, manual numbering, manual entry of test items, and secondary entry of test results during instrument operation were eliminated. Meanwhile, various errors caused by manual operation were also resolved, thereby improving working efficiency and laboratory test quality.
(2) Set up relevant prompt functions: According to the actual situation in the department, real-time specimen monitoring software was created to display upcoming timeout items (within five minutes) and timeout items (sound alarm) with the aid of a display screen and audio installed in a conspicuous position.
(3) Automatic reports of the items with critical values and monitoring of the whole process: After the critical value report system was optimized, inspectors rechecked the test results in cases with prompts on items with critical values. After the test results were checked, they were manually entered into the LIMS system and sent to the prescriber. If the recipient failed to accept the notification within the specified time, an alarm prompt was sent to the target computer. Manual notification by telephone was performed if the recipient failed to accept the notification within five minutes of the alarm prompt. These measures eliminated human error and timeouts in the manual reporting of items with critical values.
(4) IT-based optimization: The laboratory test items were optimized according to clinical practice. The blood collection tubes were integrated to reduce the volume of blood taken from patients and improve test efficiency. Additionally, problems encountered during daily operations were optimized individually, thereby contributing to user-friendly operation and close connection to the LIMS system (Table 2). The related process is presented in Fig. 2.
|
Observation indicators
In this study, relevant evaluation indicators were compared before and after optimization:
- Laboratory test report time (outpatients) = Time when the test result is reported - Time when the specimen is collected
- Laboratory test report time (inpatients) = Time when the test result is reported - Time when the specimen is accepted
- Laboratory test report time limit coincidence rate = Number of laboratory test reports within the specified time limit/Total number of laboratory test reports × 100%
- Laboratory test report error rate = Number of laboratory test reports with errors / Total number of laboratory test reports × 100%
Additionally, an anonymous satisfaction survey was performed on all employees involved in the emergency laboratory test process. The Management System of Quality Monitoring Indicators of Medical Community was developed in accordance with the standards outlined in the Joint Commission International Accreditation Standards for Hospitals (6th Edition).[15] Subsequently, 128 patients were randomly selected each month for the satisfaction survey. Satisfaction was evaluates as such:
- Satisfaction = Number of patients that are very satisfied or satisfied / Total number of patients in the satisfaction survey × 100%
Statistical analysis
Data processing was performed using SPSS 26.0, and the Kolmogorov-Smimov normality test was used to analyze the data distribution. The two-sample independent t-test was performed to make comparisons between both groups. Normally distributed measurement data were expressed using x̄ ± s, while non-normally distributed measurement data were expressed using M (IQR). Moreover, the rank sum test was used for comparisons between groups that did not conform to normal distribution, and the chi-square test was performed on the comparison of rates. P < 0.05 indicated that there was a significant statistical difference.
Results
Changes in the emergency laboratory test report time before and after IT-based optimization
After IT-based optimization was implemented, the median emergency laboratory test report time in each month from January to September 2022 was shorter than in the corresponding months from January to September 2021 (i.e., January 2022 vs. January 2021), with statistically significant differences. After IT-based optimization, the overall emergency laboratory test report time was also shorter than before optimization, and the difference between the groups was significant (P < 0.05), as indicated by Table 3.
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Changes in the emergency laboratory test report time limit coincidence rate before and after IT-based optimization
After IT-based optimization, the total number of laboratory test items was 259,651 and the emergency laboratory test report time limit coincidence rate was 99.03%. In contrast, before optimization, the total number of laboratory test items was 222,139 and the emergency laboratory test report time limit coincidence rate was 98.77%. The values of both indicators after IT-based optimization were higher than before IT-based optimization, and there was a significant difference in the emergency laboratory test report time limit coincidence rate (Table 4).
| ||||||||||||||||||||||||
Changes in the emergency laboratory test report error rate before and after IT-based optimization
Before IT-based optimization, there were 222,139 laboratory test items in total, with errors occurring in 17 laboratory test items, equating to 0.77‱. Specifically, there were five flawed test items caused by manual input errors, eight mistakes due to invalid symbol reports, two errors caused by poor specimen collection, one item with incorrect results caused by condensation, and one test item where CK-MB = 0. After IT-based optimization, there were 259,651 laboratory test items in total, and errors only occurred in four laboratory test items, accounting for 0.15‱. Specifically, there was one faulty laboratory test item in routine fecal microscopic tests, one error concerning a higher D-dimer level caused by poor specimen collection, and two mistakes related to low RBC (Red Blood Cell, RBC) levels caused by condensation. After the implementation of IT-based optimization, the emergency laboratory test report error rate decreased considerably, and there was a significant difference in this indicator between the two groups (Tables 5 and 6).
| ||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||
Comparison of patient and employee satisfaction before and after IT-based optimization
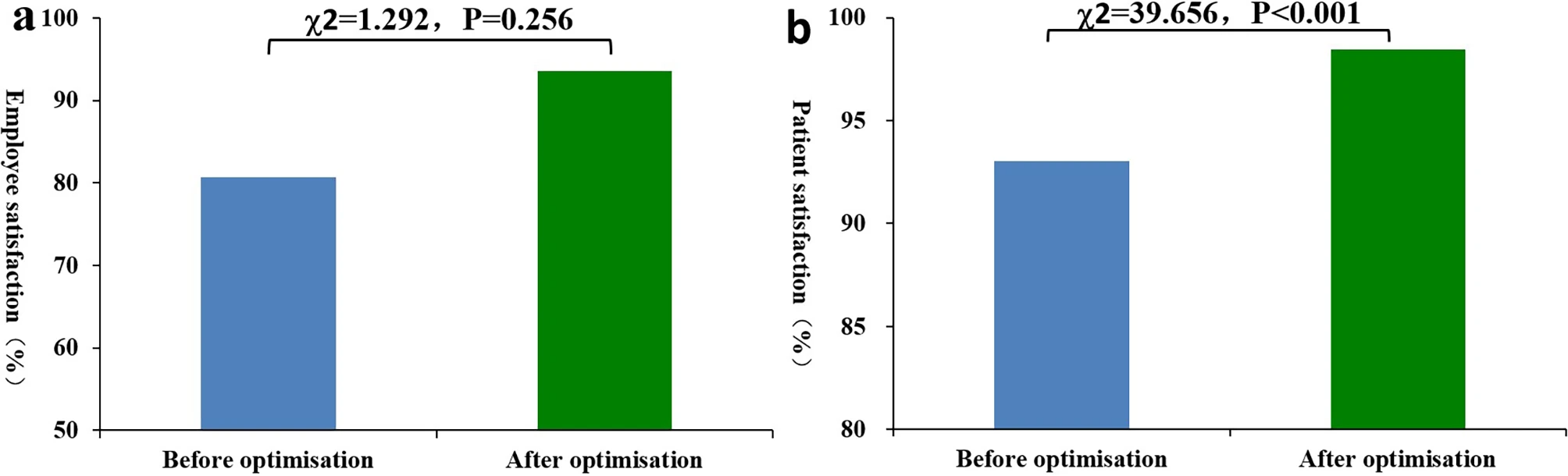
The satisfaction levels of employees involved in emergency laboratory tests (rated very satisfied or satisfied) increased from 80.65 to 93.55% after the introduction of IT-based optimization, but the difference was not statistically significant (P = 0.256). However, the number of very satisfied employees increased by 29.03% (from 54.84 to 83.87%) after IT-based optimization, and the difference was statistically significant (P = 0.025). After IT-based optimization, patient satisfaction (very satisfied or satisfied) increased from 93.06 to 98.44%, exhibiting a statistically significant difference (P < 0.001). Additionally, after IT-based optimization, the number of employees and patients who were very satisfied with the emergency laboratory tests increased significantly (employees: 17 occurring before and 26 after IT-based optimization; (patients: 940 occurring before and 1044 after IT-based optimization), and the difference was statistically significant (employees: P = 0.025, patients: P < 0.001). After IT-based optimization, the number of patients who were satisfied, generally satisfied, and dissatisfied decreased substantially, and the differences were also statistically significant (P = 0.003, P < 0.001, P < 0.001). However, for these three satisfaction levels, there was no significant difference in the satisfaction of employees involved in emergency laboratory tests before and after IT-based optimization (P = 0.096, P < 0.554, P < 0.554). See Tables 7 and 8, and Fig. 3.
| ||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||
|
Discussion
To obtain precise and accurate medical test results, most laboratories highlight analytical techniques and test quality. However, since clinicians also pay attention to service quality and the timeliness of laboratory test reports, they have stricter requirements for emergency laboratory test reports.[16][17] As specified in ISO 15189 Medical laboratories: Requirements for quality and competence[7], laboratory quality management encompasses management of the authenticity and reliability of test results and also covers the management of various factors that may affect these results. The TAT of specimens is a crucial factor that affects the quality of laboratory tests. Currently, TAT is regarded as an observation indicator for continuous quality improvement in numerous clinical laboratories around the world.[9][18] With the rapid advancement and popularization of IT, there have been tremendous improvements in the function of the hospital information system (HIS)[19] and clinical laboratory information system (LIS)[20] in hospitals at all levels. As a result, management of the conventional laboratory test process (before, during, and after analysis) has been continually improved. If the most advanced IT can be applied to medical laboratory testing, the quality and speed of these tests may be greatly improved. Using information management technology to analyze and monitor the time nodes of key stages during the inspection process enhances effectiveness and controllability. As a result, work efficiency and inspection quality are improved.
As a county-level general hospital (tertiary second-class hospital), our institution placed significant emphasis on continuous quality improvement during the re-evaluation process for tertiary second-class hospitals in 2021. Additionally, in 2013, our hospital relocated to its current site, which included a new hospital area equipped with relevant facilities. It is worth noting that by this time, many of the instruments in the clinical laboratory had reached their scheduled retirement dates and required systematic replacement. Due to said circumstances, the PDCA method was adopted to analyze relevant problems first, with a focus on improving the quality of emergency laboratory test reports. Subsequently, the PDCA tool was employed to identify the foundational framework of emergency laboratory test reports, particularly focusing on the aspect of delayed information construction. Building upon this foundation, a multifaceted approach was adopted, with IT as the focal point, to optimize the emergency laboratory test process. This optimization encompassed various comprehensive methods, including personnel management, 7 S management, equipment innovation, and alignment with hospital values, all aimed at enhancing the quality of emergency laboratory test reports. While this study indeed emphasizes the optimization of IT, it is crucial to note that the conclusion should not be solely based on the improvement of IT alone. The enhancement in the quality of emergency laboratory test reports primarily results from the comprehensive measures implemented through IT-based initiatives.
In this study, IT methods were employed to optimize each step in the emergency laboratory test process (Table 2). Even with an increased number of specimens, the laboratory test report time was still reduced by 16.67% (from 30 minutes [median] to 25 minutes [median]) after IT-based optimization. Additionally, the TAT was much shorter than the 68 minutes required to generate emergency biochemical test results, as reported by Fei[21] However, this discrepancy may be caused by differences in the test items. Specifically, we included all test items in this study, while only emergency biochemical test items were included in their study. The upper limit of the biochemical test report times in our laboratory was 60 minutes, while the TAT coincidence rate in our laboratory reached 98.77% before IT-based optimization. Additionally, the emergency laboratory test report time in this study was shorter than that in the study of Fei. This may be because precise management and IT were less advanced when they conducted their relevant studies. Moreover, under the premise that the number of specimens increased and the number of employees remained the same, the emergency laboratory test report time limit coincidence rate significantly improved. This finding suggests that IT played an important role in cutting the emergency laboratory test report time. Additionally, the emergency laboratory test report time limit coincidence rate after IT-based optimization in this study was also slightly higher than the 94.8% reported by Zhang et al.[22] This may be because they paid attention to analyzing the TAT coincidence rate and exploring the reasons for this discrepancy, rather than elucidating corrective measures.
Recently, to shorten the emergency laboratory test report time, point-of-care testing (POCT) has been introduced in some laboratories to replace conventional laboratory test methods, which has reduced test quality. In this study, more conventional and advanced instruments were adopted during IT-based optimization, thereby greatly reducing the emergency laboratory test report time. Meanwhile, the emergency laboratory test report error rate also fell from 0.77‱ to 0.15‱ after IT-based optimization, which eliminated emergency inspection report errors caused by the transmission of abnormal results from the equipment to the LIMS. Nevertheless, after IT-based optimization, certain defects were not eliminated, and there are still some items that need to be manually entered. As a result, errors still occur occasionally due to other influencing factors before specimen analysis. In the future, an automatic review will be performed by the HIS, and if the laboratory test results are inconsistent with the comprehensive performance of the patients, inspectors will be prompted to recheck the results, which may reduce errors. Additionally, the intrinsic property (RBC ≈ Hb×3.5) will be examined to prevent the occurrence of events such as RBC reduction caused by condensation. Thus, we maintain that the extensive application of IT in laboratory testing will significantly improve test speed and quality.
IT-based optimization shortens the emergency laboratory test report time and improves emergency laboratory test quality. Besides, the satisfaction levels of employees and patients also increase after the introduction of IT-based optimization. In this study, employee satisfaction increased from 80.65 to 93.55% after optimization, but the difference was not statistically significant. This may be due to false positives caused by the small number of employees. Specifically, the number of very satisfied employees increased by 29.03% (from 54.84 to 83.87%) after IT-based optimization. Although the number of participants was relatively small, the difference was still statistically significant, and this result confirms that employee satisfaction rose as a result of IT-based optimization. Additionally, several manual operations were replaced by automatic processing after IT-based optimization. Hence, human error was avoided and employees could pay more attention to resolving complex problems. Also, even though the total workload increases, work intensity may decrease due to workflow optimization and the implementation of informationization and automation, which may be another reason for the improvement in employee satisfaction. Based on the integration of artificial intelligence (AI) and emergency laboratory tests, emergency laboratory test quality was comprehensively enhanced. Thus, employees obtained a higher sense of achievement, which may have contributed to the upturn in employee satisfaction. After IT-based optimization, the overall satisfaction and high satisfaction of patients were greater than before IT-based optimization. Additionally, the absolute number and relative proportion of patients who were satisfied, generally satisfied, or dissatisfied also decreased significantly after IT-based optimization. Reduced emergency laboratory test report times implied shorter waiting times, which may have relieved the anxiety of patients. Additionally, improved emergency test quality contributed to higher faith in the laboratory test results, which may have enhanced patient recognition of the test results. Therefore, the emergency laboratory test report time, test quality, and employee and patient satisfaction are all dependent on the overall quality of the emergency laboratory tests, which can be further improved through comprehensive optimization.
Owing to constraints in resources, this study was configured as a single-center investigation, and the randomized controlled trial approach was not employed. Consequently, there exists the potential for some bias in the outcomes of this study, specifically in relation to the enhancement of emergency laboratory test report quality and the reduction of emergency laboratory test report time. Hence, a multi-center study is needed for further verification. Within this study, the IT-driven optimization of emergency laboratory tests resulted in improvements not only in the quality of emergency laboratory test reports but also in the reduction of turnaround times for these reports. The production of precise and swift examination reports played a pivotal role in ensuring that emergency patients received effective care within the optimal rescue window. Moreover, it contributed to a reduction in the waiting period for patients and their families at the hospital. This contributed to alleviating their anxiety, thereby leading to a comprehensive improvement in their medical experience and satisfaction. Furthermore, the decreased work intensity and error rate among medical professionals engaged in emergency laboratory tests can elevate their sense of professional pride. As a result, the development of advanced emergency laboratory test capabilities is highly commended by clinicians, patients, and laboratory technicians.
Conclusion
With the recent rapid advances in IT, the integration of IT into laboratory testing has become a popular research direction. The automation and standardization of most stages in emergency laboratory testing can be realized by IT, which reduces the workload of employees and improves emergency laboratory test quality. However, the application of advanced technology in emergency laboratory testing requires the cooperation of IT engineers and inspectors. Thus, it is necessary to understand the cutting-edge knowledge related to information technology and laboratory testing to combine advanced theory with practical functions. Furthermore, training projects should also be implemented to promote the application of advanced technology, thereby maximizing the advantages of IT. On that basis, the emergency laboratory test report time can be shortened, emergency laboratory test quality can be enhanced, and employee and patient satisfaction can also be improved. Moreover, the IT-based optimization of the emergency test process should be conducted according to thorough evaluations of defects in existing laboratory processes. Meanwhile, it should be noted that IT-based optimization is also a cumulative process. After the occurrence of any problems, it is necessary to conduct a comprehensive analysis to identify feasible solutions that contribute to optimization.
Acknowledgements
We would like to acknowledge the hard and dedicated work of all the staff that implemented the intervention and evaluation components of the study.
Author contributions
Conception and design of the research: Mei Li Guo and Liang Zhang. Acquisition of data: Zhang Mei Luo. Analysis and interpretation of the data: Yin Jiang Lv and Shui Fu. Statistical analysis: Zhen Hua Liu. Obtaining financing: Mei-Li Guo. Writing of the manuscript: Liang Zhang. Critical revision of the manuscript for intellectual content: Yin Jiang Lv, Shui Fu and Mei-Li Guo. All authors read and approved the final manuscript.
Funding
Hangzhou Agricultural and Social Development Project (grant number: 20220919Y106).
Data availability
We declared that materials described in the manuscript, including all relevant raw data, will be freely available to any scientist wishing to use them for non-commercial purposes, without breaching participant confidentiality.
Ethics approval and consent to participate
This study was conducted with approval from the Ethics Committee of Linping Campus, The Second Affiliated Hospital of Zhejiang University School of Medicine (No.2021005). This study was conducted in accordance with the declaration of Helsinki. Written informed consent was obtained from all participants.
Competing interests
The authors declare no competing interests.
References
- ↑ Chinese Medical Association Laboratory Medicine Branch; Chinese Physician Association Emergency Physician Branch; Chinese People's Liberation Army Emergency Medicine Professional Committee. "Expert consensus on the development of standardization of emergency medical laboratory capacity in Chinese". Chinese Journal of Laboratory Medicine. doi:10.3760/cma.j.issn.1009-8158.2020.01.001. https://rs.yiigle.com/cmaid/1177751.
- ↑ Quadflieg, Lina T. M.; Beckers, Stefan K.; Bergrath, Sebastian; Brockert, Ann-Katrin; Schröder, Hanna; Sommer, Anja; Brokmann, Jörg C.; Rossaint, Rolf et al. (22 October 2020). "Comparing the diagnostic concordance of tele-EMS and on-site-EMS physicians in emergency medical services: a retrospective cohort study" (in en). Scientific Reports 10 (1): 17982. doi:10.1038/s41598-020-75149-8. ISSN 2045-2322. PMC PMC7581718. PMID 33093557. https://www.nature.com/articles/s41598-020-75149-8.
- ↑ Shi, Xiaofeng; Bao, Jiating; Zhang, Haili; Wang, Hao; Wang, Yu; Li, Lei; Hou, Peter (1 March 2020). "Emergency medicine in China: A review of the history of progress and current and future challenges after 40 years of reform" (in en). The American Journal of Emergency Medicine 38 (3): 662–669. doi:10.1016/j.ajem.2019.11.008. https://linkinghub.elsevier.com/retrieve/pii/S0735675719307478.
- ↑ Schmutz, Thomas; Ribordy, Vincent; Exadaktylos, Aristomenis K; Carron, Pierre-Nicolas (1 August 2021). "Emergency medicine in Switzerland: a laboratory for professional experimentation" (in en). European Journal of Emergency Medicine 28 (4): 264–265. doi:10.1097/MEJ.0000000000000816. ISSN 0969-9546. https://journals.lww.com/10.1097/MEJ.0000000000000816.
- ↑ Weingart, Gregory S.; Jordan, Phillip; Yee, Kei‐Lwun; Green, Lauren (1 February 2021). "Utility of laboratory markers in evaluating for acute compartment syndrome in the emergency department" (in en). Journal of the American College of Emergency Physicians Open 2 (1): e12334. doi:10.1002/emp2.12334. ISSN 2688-1152. PMC PMC7819267. PMID 33521785. https://onlinelibrary.wiley.com/doi/10.1002/emp2.12334.
- ↑ Cruz, Andrea T. (1 December 2018). "Indications and Interpretation of Common Laboratory Assays in the Emergency Department" (in en). Pediatric Clinics of North America 65 (6): 1191–1204. doi:10.1016/j.pcl.2018.07.005. https://linkinghub.elsevier.com/retrieve/pii/S0031395518301020.
- ↑ 7.0 7.1 Dawande, Pratibha P; Wankhade, Rashmi S; Akhtar, Faizan I; Noman, Obaid (6 September 2022). "Turnaround Time: An Efficacy Measure for Medical Laboratories" (in en). Cureus. doi:10.7759/cureus.28824. ISSN 2168-8184. PMC PMC9535613. PMID 36225468. https://www.cureus.com/articles/108313-turnaround-time-an-efficacy-measure-for-medical-laboratories.
- ↑ "ISO 15189:2022 Medical laboratories: Requirements for quality and competence". International Organization for Standardization. December 2022. https://www.iso.org/standard/76677.html.
- ↑ 9.0 9.1 Lee, Seunghoo; Yoon, Sangpil; Lee, Woochang; Chun, Sail; Min, Won‐Ki (1 October 2022). "Strategies to shorten turnaround time in outpatient laboratory" (in en). Journal of Clinical Laboratory Analysis 36 (10): e24665. doi:10.1002/jcla.24665. ISSN 0887-8013. PMC PMC9550964. PMID 36036784. https://onlinelibrary.wiley.com/doi/10.1002/jcla.24665.
- ↑ Kaushik, Nitin; Khangulov, Victor S.; O’Hara, Matthew; Arnaout, Ramy (20 April 2018). "Reduction in laboratory turnaround time decreases emergency room length of stay" (in English). Open Access Emergency Medicine 10: 37–45. doi:10.2147/OAEM.S155988. PMC PMC5916382. PMID 29719423. https://www.dovepress.com/reduction-in-laboratory-turnaround-time-decreases-emergency-room-lengt-peer-reviewed-fulltext-article-OAEM.
- ↑ Wang, Hao; Wang, Xinyue; Wang, Kouqiong; Duan, Xincen; Jiang, Wenhai; Tang, Bin; Pan, Baishen; Wang, Beili et al. (1 April 2022). "Evaluation of a cardiac troponin process flow at the chest pain center with the shortest turnaround time" (in en). Journal of Clinical Laboratory Analysis 36 (4): e24335. doi:10.1002/jcla.24335. ISSN 0887-8013. PMC PMC8993626. PMID 35263018. https://onlinelibrary.wiley.com/doi/10.1002/jcla.24335.
- ↑ Wu, Li-Feng; Zhuang, Guo-Hua; Hu, Qi-Lei; Zhang, Liang; Luo, Zhang-Mei; Lv, Yin-Jiang; Tang, Jian (1 December 2022). "Using information technology to optimize the identification process for outpatients having blood drawn and improve patient satisfaction" (in en). BMC Medical Informatics and Decision Making 22 (1): 61. doi:10.1186/s12911-022-01799-5. ISSN 1472-6947. PMC PMC8915497. PMID 35272653. https://bmcmedinformdecismak.biomedcentral.com/articles/10.1186/s12911-022-01799-5.
- ↑ Fu, Shui; Wu, Xian-Guo; Zhang, Liang; Wu, Li-Feng; Luo, Zhang-Mei; Hu, Qi-Lei (1 July 2021). "Service Quality Improvement of Outpatient Blood Collection by Lean Management" (in en). Patient Preference and Adherence Volume 15: 1537–1543. doi:10.2147/PPA.S320163. ISSN 1177-889X. PMC PMC8277444. PMID 34276209. https://www.dovepress.com/service-quality-improvement-of-outpatient-blood-collection-by-lean-man-peer-reviewed-fulltext-article-PPA.
- ↑ Jovičić, Snežana; Siodmiak, Joanna; Alcorta, Marta Duque; Kittel, Maximillian; Oosterhuis, Wytze; Aakre, Kristin Moberg; Jørgensen, Per; Palicka, Vladimir et al. (26 March 2021). "Quality benchmarking of smartphone laboratory medicine applications: comparison of laboratory medicine specialists’ and non-laboratory medicine professionals’ evaluation" (in en). Clinical Chemistry and Laboratory Medicine (CCLM) 59 (4): 693–699. doi:10.1515/cclm-2020-0869. ISSN 1434-6621. https://www.degruyter.com/document/doi/10.1515/cclm-2020-0869/html.
- ↑ "Joint Commission International Accreditation Standards for Hospitals, Sixth Edition". Joint Commission International. 1 July 2017. https://www.jointcommissioninternational.org/-/media/jci/jci-documents/accreditation/hospital-and-amc/learn/jci_standards_only_6th_ed_hospital.pdf?db=web&hash=E2D36799998C7EE27C59CFF3131EE0A7.
- ↑ Steindel, Steven J.; Howanitz, Peter J. (1 July 2001). "Physician Satisfaction and Emergency Department Laboratory Test Turnaround Time" (in en). Archives of Pathology & Laboratory Medicine 125 (7): 863–871. doi:10.5858/2001-125-0863-PSAEDL. ISSN 1543-2165. https://meridian.allenpress.com/aplm/article/125/7/863/453104/Physician-Satisfaction-and-Emergency-Department.
- ↑ Groothuis, Siebren; Goldschmidt, Henk MJ; Drupsteen, Esther J; de Vries, Jules CM; Hasman, Arie; van Merode, Godefridus G (1 May 2002). "Application of computer simulation analysis to assess the effects of relocating a hospital phlebotomy department" (in en). Annals of Clinical Biochemistry: International Journal of Laboratory Medicine 39 (3): 261–272. doi:10.1258/0004563021901964. ISSN 0004-5632. http://journals.sagepub.com/doi/10.1258/0004563021901964.
- ↑ Lijuan, Y.U.; Sancheng, C.A.O.; Shouzhen, W.U. et al. (2018). "Effect of Shenmai injection combined with immune-enhanced enteral nutrition on hyperlipidemia acute pancreatitis". Laboratory Medicine and Clinic 15 (15): 2757–9. doi:10.3969/j.issn.1672-9455.2018.15.021. http://www.chinadoi.cn/portal/mr.action?doi=10.3969/j.issn.1672-9455.2018.15.021.
- ↑ Karitis, Konstantinos; Gallos, Parisis; Triantafyllou, Ioannis S.; Plagianakos, Vassilis (18 November 2021), Delgado, Jaime; Benis, Arriel; de Toledo, Paula et al.., eds., "Chios Hospital Information System Assessment", Studies in Health Technology and Informatics (IOS Press), doi:10.3233/shti210837, ISBN 978-1-64368-236-5, https://ebooks.iospress.nl/doi/10.3233/SHTI210837. Retrieved 2024-05-07
- ↑ Sepulveda, Jorge L.; Young, Donald S. (1 August 2013). "The Ideal Laboratory Information System" (in en). Archives of Pathology & Laboratory Medicine 137 (8): 1129–1140. doi:10.5858/arpa.2012-0362-RA. ISSN 1543-2165. http://meridian.allenpress.com/aplm/article/137/8/1129/65418/The-Ideal-Laboratory-Information-System.
- ↑ Fei, Y. (2017). "Improvement of the turnaround times of STAT biochemistry test in clinical laboratory". Chinese Journal of Laboratory Medicine 40 (07): 535–9. doi:10.3760/cma.j.issn.1009-9158.2017.07.013. http://www.chinadoi.cn/portal/mr.action?doi=10.3760/cma.j.issn.1009-9158.2017.07.013.
- ↑ Zhang, H.; Xiong, L.; Wang, J. et al. (2017). "Management and practice of intra-laboratory Turn-Around-Time in emergency testing laboratory". International Journal of Laboratory Medicine 38 (8): 1079–81. https://www.alljournals.cn/view_abstract.aspx?pcid=A9DB1C13C87CE289EA38239A9433C9DC&cid=2F92804C2B75A393&jid=FE8D53848BDE4C8E1E27C01A5CB8BF09&aid=647AE7297532E741FA1C5A77EBDA8E58&yid=FA004A8A4ED1540B&vid=&iid=&sid=&eid=&from_absract=1.
Notes
This presentation is faithful to the original, with only a few minor changes to presentation. In some cases important information was missing from the references, and that information was added.