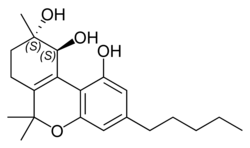
Cannabitriol
From LIMSWiki
Jump to navigationJump to searchThe printable version is no longer supported and may have rendering errors. Please update your browser bookmarks and please use the default browser print function instead.
 | |
| Clinical data | |
|---|---|
| Other names | (+)-CBT, (S,S)-9,10-Dihydroxy-Δ6a(10a)-THC |
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C21H30O4 |
| Molar mass | 346.467 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Cannabitriol (CBT) is a phytocannabinoid first isolated in 1966,[1][2][3] an oxidation product of tetrahydrocannabinol (THC) which has been identified both as a trace component of cannabis and as a metabolite in cannabis users.[4] Its pharmacology has been little studied, though it has been found to act as an antiestrogen and aromatase inhibitor.[5][6]
See also
References
- ^ Obata Y, Ishikawa Y (June 1966). "Studies on the constituents of hemp plant (Cannabis sativa L.) Part III. Isolation of a gibbs-positive compound from Japanese hemp". Agricultural and Biological Chemistry. 30 (6): 619–620. doi:10.1080/00021369.1966.10858651.
- ^ Chan WR, Magnus KE, Watson HA (March 1976). "The structure of cannabitriol". Experientia. 32 (3): 283–4. doi:10.1007/BF01940792. PMID 1253891. S2CID 2679030.
- ^ Elsohly MA, El-Feraly FS, Turner CE (1977). "Isolation and characterization of (+)-cannabitriol and (-)-10-ethoxy-9-hydroxy-delta 6a[10a]-tetrahydrocannabinol: two new cannabinoids from Cannabis sativa L. extract". Lloydia. 40 (3): 275–80. PMID 895385.
- ^ White RM (January 2018). "Instability and poor recovery of cannabinoids in urine, oral fluid, and hair". Forensic Science Review. 30 (1): 33–49. PMID 29273570.
- ^ Baroi S, Saha A, Bachar R, Bachar SC (June 2020). "Cannabinoid as Potential Aromatase Inhibitor through Molecular Modeling and Screening for Anti-Cancer Activity". Dhaka University Journal of Pharmaceutical Sciences. 19 (1): 47–58. doi:10.3329/dujps.v19i1.47818. S2CID 225712476.
- ^ Kikiowo B, Ogunleye AJ, Iwaloye O, Ijatuyi TT, Adelakun NS, Alashe WO (2021). "Induced Fit Docking and Automated QSAR Studies Reveal the ER-α Inhibitory Activity of Cannabis sativa in Breast Cancer". Recent Patents on Anti-Cancer Drug Discovery. 16 (2): 273–284. doi:10.2174/1574892816666210201115359. PMID 33563181. S2CID 231865568.
External links
Notes
This article is a direct transclusion of the Wikipedia article and therefore may not meet the same editing standards as LIMSwiki.









