Journal:The cannabis terpenes
| Full article title | The cannabis terpenes |
|---|---|
| Journal | Molecules |
| Author(s) | Sommano, Sarana R.; Chittasupho, Chuda; Ruksiriwanich, Warintorn; Jantrawut, Pensak |
| Author affiliation(s) | Chiang Mai University |
| Primary contact | Email: sarana dot s at cmu dot ac dot th |
| Editors | Valgimigli, Luca |
| Year published | 2020 |
| Volume and issue | 25(24) |
| Article # | 5792 |
| DOI | 10.3390/molecules25245792 |
| ISSN | 1420-3049 |
| Distribution license | Creative Commons Attribution 4.0 International |
| Website | https://www.mdpi.com/1420-3049/25/24/5792/htm |
| Download | https://www.mdpi.com/1420-3049/25/24/5792/pdf (PDF) |
|
|
This article should be considered a work in progress and incomplete. Consider this article incomplete until this notice is removed. |
Abstract
Terpenes are the primary constituents of essential oils and are responsible for the aroma characteristics of the Cannabis plant. Together with cannabinoids, terpenes illustrate a potential synergic and/or entourage effect, with their interactions having only been speculated on for the last few decades. Hundreds of terpenes have been identified that additionally add to the overall cannabis sensory experience, contributing largely to the consumer’s experiences, as well as the market price. These terpenes also enhance many therapeutic efforts, especially as aromatherapy. To shed light on the importance of terpenes in the cannabis industry, the purpose of this review is to morphologically describe sources of cannabis terpenes and to explain the biosynthesis and diversity of terpene profiles in different cannabis chemotypes.
Introduction
Cannabis sativa L. or Cannabis is a herbaceous annual that has a long history of use around the world as fiber, food, oil, and medicine. Depending on the purpose of utilization, cannabis can be called by different names, for example, “hemp” as a fiber and textile crop, and “recreational cannabis,” also known in the United States as "marijuana." Aside from its quality as an industrial textile, the psychoactive properties have placed a stigma on Cannabis plants as an illicit drug, with many informal names including pot, dope, grass, weed, Mary Jane, bud, hash, bhang, kef, ganja, locoweed, reefer, doob, spliff, toke, and roach. It has been forbidden to grow in many countries due to its psychoactive constituents.[1] From a medical perspective, many recent studies have advised that an increase in cannabis use is associated with psychiatric symptoms, including depression and anxiety.[2][3] However, many users still exclusively endorse its recreational purpose.[4][5] As a result, there has been a strong movement toward correcting negative attitudes about Cannabis, and attempts have been made in trying to remove this plant from narcotic lists. In Thailand, for instance, as of 2020, cannabis strains such as กัญชง (hemp) and กัญชา (marijuana) are legally grown for industrial fiber or medicinal purposes based on the controlled level of active metabolites, including cannabinoids such as Δ9–tetrahydrocannabinol (THC) and cannabidiol (CBD).[6] While primary focus has been paid to the bioactive functions of the cannabinoids, the hydrocarbon terpenes could also potentially offer interesting entourage effects that could ideally synergize or downstream their effects.[7] Eminently, with the rise in the legal cannabis industry, interest has grown around cannabis terpenes as they contribute many of the different aromatic characteristics that influence the diverse varieties of cannabis strains.[8]
Within the scope of this review, we provide the general background history of cannabis discovery and the importance of terpenes. The taxonomy and morphology of cannabis, particularly in regards to the localization of its terpenes, are discussed. More importantly, the chemistry, biosynthesis, and diversity of terpenes in different cannabis genotypes are of major interest in this review.
The cannabis discovery and its importance as a source of terpene
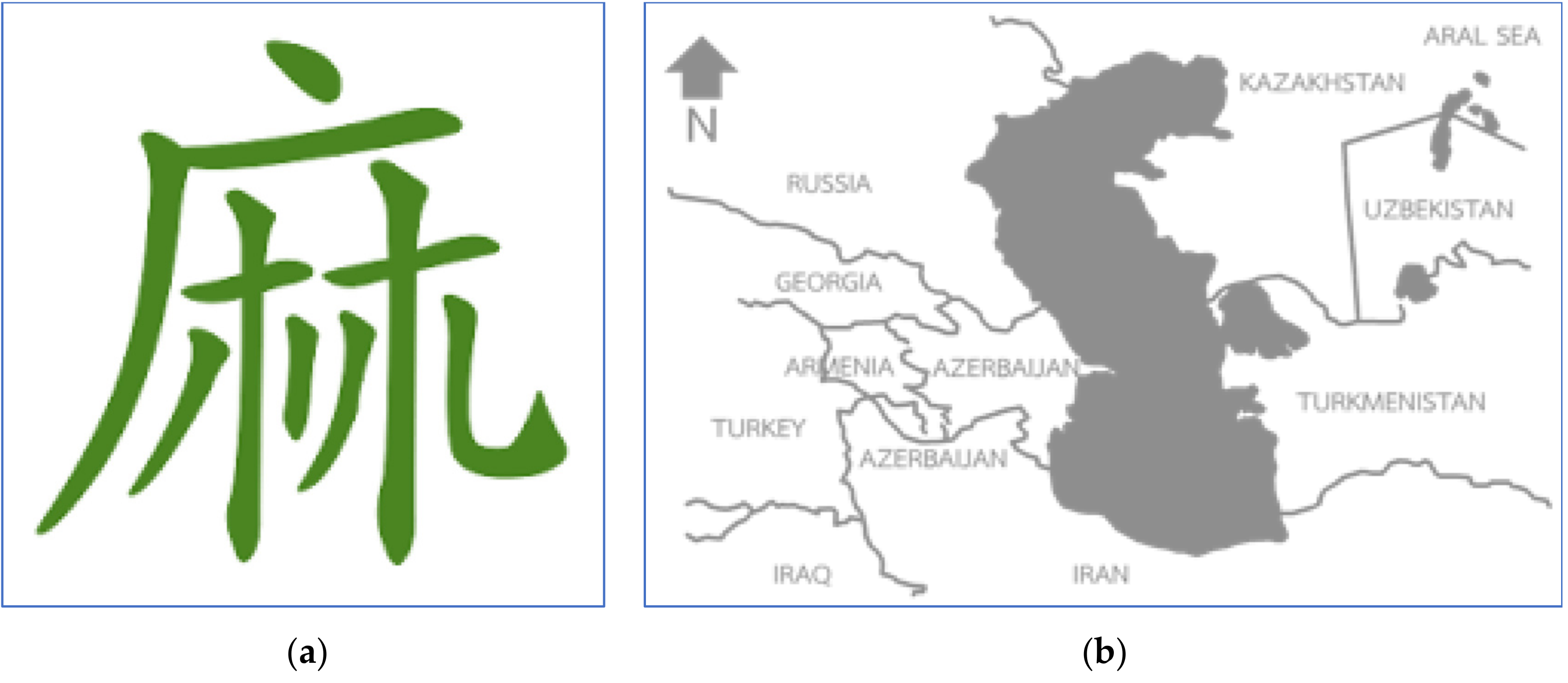
Cannabis has a long history dating back approximately to just after the Ice Age, as cord and textile scraps made of cannabis fiber have been found in historic caves in the Czech Republic.[9][10] In the nineteenth century, the plant was recorded as originating near the southern area of the Caspian Sea near Iran (Figure 1b).[11][12] It was later confirmed by the chemotaxonomy of the essential oil from cannabis of diverse origins, and most of the cannabis phenotypes collected around the globe had chemical ingredients similar to those of Central Asian origin.[13] In previous days, it was known as the original fiber plant in Asian culture. Seed and seed oil extracts were also used as food.[11][14] The first record of cannabis in the literature in China can be dated to approximately 5,000 years ago, as written by emperor Chen Nong, who was then known as the father of Chinese agriculture. The Chinese alphabet's “Ma” was created using the mimic of the cannabis drying process (Figure 1a). The letter was adjusted to describe the male plant, “his” separated from the female plant, “chu” for the quality of the fiber.[15][16] In 500 BC, the use of cannabis spread worldwide from Asia to Europe and to Africa through the silk road. In the nineteenth century, hemp was popular in the western world as a fiber crop that had superior qualities.[17][18] It is not too surprising therefore that cannabis is today known as an ideal plant in terms of sustainability. In a period of rapid industrial growth, hemp became the industrial crop as countries raced against one another toward modernity. In addition to the world textile industry, the plant began to be known for its medical uses. It was believed that during the nineteenth century, there were thousands of cannabis medicines available, produced by more than 280 manufacturers.[19] However, the growth and interest in this fiber crop crashed after the attempt to add it to the narcotic list with opium during the 1925 International Opium Convention in Geneva.[19]
|
The first cannabinoid isolates used for medicinal purposes were produced in Czechoslovakia, and CBD was fully characterized for the first time in 1963, followed by the psychoactive THC in the following year.[20][21][22] The discovery of cannabinoid receptors, CB1 and CB2, together with the full comprehension of the endocannabinoid system, helped us recognize the medicinal benefits of this plant.[23][24] In 1942, Simonsen and Todd[25] were the first researchers to separate terpene fractions from cannabinoids in the literature, and p-Cymene was reported as a main constituent from Egyptian hashish. Only in the past years have the terms "synergic" and "entourage effect" appeared in speculations by chemists all over the world in relation to cannabis compounds, included the terpenes.[26] The proposed synergies were first described as routes for the molecular regulation of endogenous cannabinoid activity.[27] Russo[28] later proposed the unique therapeutic effect of cannabis terpenes that possibly play a role on the entourage effects of the medicinal properties of the cannabinoids. This phytocannabinoid-terpenoid synergy could potentially enhance the treatments of pain, inflammation, depression, anxiety, addiction, epilepsy, cancer, fungal, and bacterial infections.[7][28][29][30][31]
Taxonomy and localization of cannabis terpenes
Cannabis belongs to the small family of flowering plants known as Cannabaceae, which includes hop and Celtis berry. It is a dicotylate angiosperm that gives incomplete (lacking of petals) and also imperfect flower types with the separated sexual organs. The flower bears only pistils, known as pistillate flowers (or female flowers), and those with only the stamens are called staminate (or male flowers). In nature, Cannabis produces either male or female flowers (dioecious); however, under short-day conditions, it may give both male and female flowers or monoecious. Plants are able to grow as high as three meters, or smaller depending on the varieties and the conditions of growth. The flowers often initiate as groups of flowers or as an axillary bud (Figure 2a and 2b). The stem consists of the outermost layer of the epidermis, which is thin and coarse, and the primary and secondary layers that provide the better fiber quality. The innermost, which is the lignified secondary blast fiber, give the best fiber quality.[32][33]
|
Leaves are palmate with five to seven leaflets. The male flower (Figure 3a) has no petals, usually with five yellowish tepals, and five anthers yielding pollen. The female flower (Figure 3b) had a single-ovulate and is enriched with trichome structures, which are the localization points of cannabinoids and terpenes, as shown in Figure 3c. These terpenes are responsible for the defense and interaction with herbivores and pests.
|
Taxonomists classified Cannabis plants into three species in the early days: (i) C. sativa Linnaeus, (ii) C. indica Lamarck, and (iii) C. ruderalis.[34][35] Today, many researchers are convinced that Cannabis that grows commercially is C. sativa L., but the subvariety “sativa” should be known as hemp and the subvariety “indica” should be called recreational cannabis or marijuana. The differences in these sub-varieties are shown in Table 1.
| |||||||||||||||||||||||||||
The most usable part for hemp is the stem in particular, while parts usually with trichomes are the most usable for cannabis. The level of THC is graded as >2% of dry weight, and flowers give a much higher terpene content, which becomes sticky to the touch.[36] Some researchers do not agree with the separation of the two by the chemical compositions. Morphologically, the leaf of cannabis is broader and the color is darker compared to that of hemp. Many recent studies have attempted to separate the combinations of terpene composition in several species of Cannabaceae where it is apparent that hemp and the Indica cannabis are closely related. For example, hemp can also yield terpene profiles similar to those of marijuana.[37]
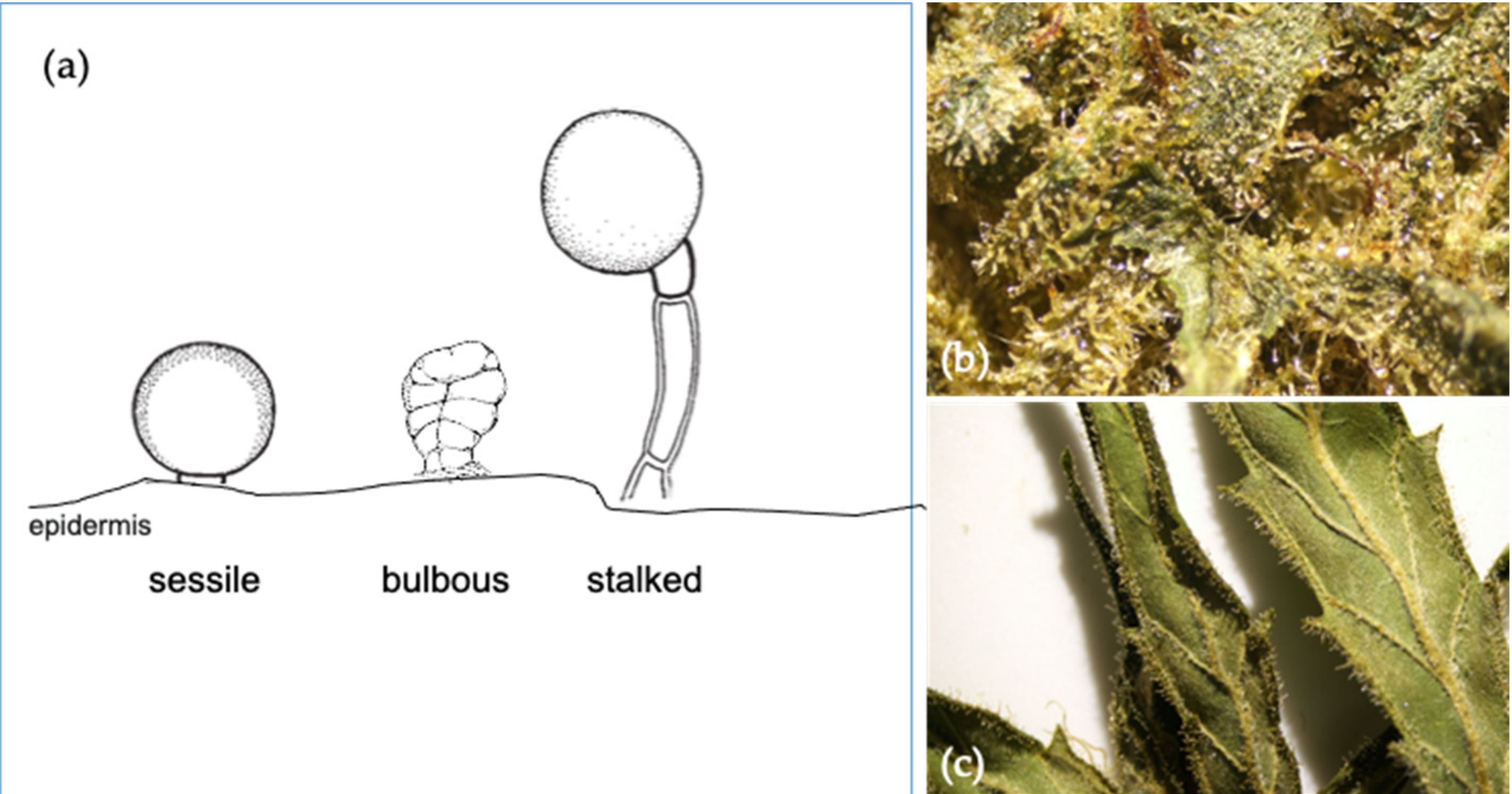
Three types of glandular trichomes are characterized based upon their surface morphology, namely bulbous, sessile, and stalked (Figure 4a).[38] Bulbous trichomes are the smallest, while sessile trichomes appear on the epidermis with a short stalk and a globose head comprised of a multicellular disc of secretory cells and a subcuticular metabolite storage cavity. Similarly, the stalked trichomes are slightly larger with a globose head elevated above the epidermal surface by a multicellular stalk.[39] In cannabis, the sessile and stalked trichomes differ not only in morphology, but they also have distinct fluorescent properties, number of cells in their secretory disc, and terpene profiles.[40] The stalked glandular trichomes of mature flowers have a globular head consisting of an enlarged disc greater than eight secretory cells known to be rich in cannabinoids and monoterpenes (Figure 4b). The sessile trichomes are mainly found on sugar leaves (Figure 4c). They have eight secretory cells that produce fewer cannabinoids and higher proportions of sesquiterpenes.
|
To separate the trichomes, the flowers are usually pre-frozen or freeze-dried and then are gently rubbed on sieve mesh. The trichomes separated in this process are known as kief, which can be pressed to make hash. In Nepal, hash is hand-shaped into balls, also known as wax or “Charas.”[41] Hash oil, on the other hand, is the concentrated hash that has been dissolved in organic solvents such as alcohol, propane, or butane.[42][43] The extraction allows pigments such as chlorophylls and other contaminants to be extracted along with the terpenes, resulting in a dark green color extract. After extraction, the solvent is then removed by evaporation either by direct heat or under a vacuum, resulting in the oil product with high viscosity.
Terpene biosynthesis in cannabis
The energy required for plant growth and development derives from photosynthesis, respiration, and transpiration with O2, CO2, nutrients, and water. The energy is restored in the form of primary chemical ingredients that plants later exploit. These primary metabolites include carbohydrates, lipids, proteins, and nucleic acids. However, during cycles of growth and reproduction, plants might be challenged by stresses including hard environmental conditions, pests, and herbivores. Plants then produce different groups of compounds called secondary metabolites that are used as defenses to those challenges. For example, it can produce compounds that draw in pollinators, including birds to help them in the fertilization process or seed dispersion.[44] These compounds are produced in different forms and are exploited for their biological functionalities[45]; for example, alkaloids such as morphine and codeine in opium give psychoactive and pain relief activity to mammals. Phenolics and flavonoids found in the skins of fruits and berries possess antioxidant activity.[46] Sulfur containing compounds such as allicin in garlic can be used to reduce lipoglycerides in the blood and also have the ability to stimulate appetite.[47] Saponin glycoside in soap nuts can be used as a surfactant[48], and finally, the terpenoids, which are main ingredients found in plants containing essential oils[49], are used as food additives, and some depict psychoactive ability and aroma characteristics such as those found in cannabis.
Terpenes are hydrocarbons with small isoprene units linked to one another to form chains, while terpenoids are oxygen-containing terpenes. Four types of terpenes/terpenoids are usually found in the Cannabis plant[26]:
- monoterpenes (10C) of two isoprene units
- sesquiterpenes (15C) of three isoprene units
- diterpenes (20C) of four isoprene units
- triterpenes (30C) of six isoprene units
To date, more than 200 volatiles have been reported from the different cannabis genotypes, of which 58 monoterpenes and 38 sesquiterpenes have been characterized.[50][51][52][53] Figure 5a illustrates a chromatogram of the terpene extract from the floral tissue of cannabis. Among others, the major monoterpene components are limonene, β-myrcene, α-pinene, and linalool, with traces of α-terpinolene and tran-ocimene[54][55] (Figure 5b), while predominate sesquiterpenes are [Caryophyllene|E-caryophyllene]], caryophyllene oxide, E-β-farnesene, and β-caryophyllene.[56] The cannabinoids are biologically synthesized from diterpene structures to form phenol terpenoids, which account for almost a quarter of all metabolites.[26] Thus, the combination of the terpenes provides the unique aromas to different strains.
References
- ↑ Chaohua, Cheng; Gonggu, Zang; Lining, Zhao; Chunsheng, Gao; Qing, Tang; Jianhua, Chen; Xinbo, Guo; Dingxiang, Peng et al. (1 May 2016). "A rapid shoot regeneration protocol from the cotyledons of hemp (Cannabis sativa L.)" (in en). Industrial Crops and Products 83: 61–65. doi:10.1016/j.indcrop.2015.12.035. https://linkinghub.elsevier.com/retrieve/pii/S0926669015306245.
- ↑ Weinberger, Andrea H.; Zhu, Jiaqi; Levin, Jacob; Barrington-Trimis, Jessica L.; Copeland, Jan; Wyka, Katarzyna; Kim, June H.; Goodwin, Renee D. (1 September 2020). "Cannabis use among US adults with anxiety from 2008 to 2017: The role of state-level cannabis legalization". Drug and Alcohol Dependence 214: 108163. doi:10.1016/j.drugalcdep.2020.108163. ISSN 1879-0046. PMID 32707516. https://pubmed.ncbi.nlm.nih.gov/32707516.
- ↑ Rabiee, Rynaz; Lundin, Andreas; Agardh, Emilie; Hensing, Gunnel; Allebeck, Peter; Danielsson, Anna-Karin (1 November 2020). "Cannabis use and the risk of anxiety and depression in women: A comparison of three Swedish cohorts". Drug and Alcohol Dependence 216: 108332. doi:10.1016/j.drugalcdep.2020.108332. ISSN 1879-0046. PMID 33080503. https://pubmed.ncbi.nlm.nih.gov/33080503.
- ↑ Lloyd, Shawnta L.; Lopez-Quintero, Catalina; Striley, Catherine W. (1 November 2020). "Sex differences in driving under the influence of cannabis: The role of medical and recreational cannabis use". Addictive Behaviors 110: 106525. doi:10.1016/j.addbeh.2020.106525. ISSN 1873-6327. PMC 7443537. PMID 32711286. https://pubmed.ncbi.nlm.nih.gov/32711286.
- ↑ Turna, Jasmine; Balodis, Iris; Munn, Catharine; Van Ameringen, Michael; Busse, Jason; MacKillop, James (1 October 2020). "Overlapping patterns of recreational and medical cannabis use in a large community sample of cannabis users". Comprehensive Psychiatry 102: 152188. doi:10.1016/j.comppsych.2020.152188. ISSN 1532-8384. PMID 32653594. https://pubmed.ncbi.nlm.nih.gov/32653594.
- ↑ Theparat, C. (29 January 2020). "New rule makes it legal to grow hemp". Bangkok Post. https://www.bangkokpost.com/thailand/general/1845714/new-rule-makes-it-legal-to-grow-hemp. Retrieved 02 October 2020.
- ↑ 7.0 7.1 Koltai, Hinanit; Namdar, Dvora (1 October 2020). "Cannabis Phytomolecule 'Entourage': From Domestication to Medical Use". Trends in Plant Science 25 (10): 976–984. doi:10.1016/j.tplants.2020.04.007. ISSN 1878-4372. PMID 32417167. https://pubmed.ncbi.nlm.nih.gov/32417167.
- ↑ Booth, Judith K.; Bohlmann, Jörg (1 July 2019). "Terpenes in Cannabis sativa – From plant genome to humans" (in en). Plant Science 284: 67–72. doi:10.1016/j.plantsci.2019.03.022. https://linkinghub.elsevier.com/retrieve/pii/S0168945219301190.
- ↑ Clarke, R.; Merlin M., ed. (1 September 2013). "History of Cannabis Use for Fiber". Cannabis: Evolution and Ethnobotany. University of California Press. doi:10.1525/9780520954571-011. ISBN 978-0-520-95457-1. https://california.degruyter.com/view/title/554936.
- ↑ Fleming, M.P.; Clarke R.C. (1998). "Physical evidence for the antiquity of Cannabis sativa L.". Journal of the International Hemp Association 5 (2): 80–92. http://www.internationalhempassociation.org/jiha/jiha5208.html.
- ↑ 11.0 11.1 Li, Hui-Lin (1 October 1973). "An archaeological and historical account of cannabis in China" (in en). Economic Botany 28 (4): 437–448. doi:10.1007/BF02862859. ISSN 0013-0001. http://link.springer.com/10.1007/BF02862859.
- ↑ Li, Hui-Lin (1 July 1974). "The origin and use of cannabis in eastern asia linguistic-cultural implications" (in en). Economic Botany 28 (3): 293–301. doi:10.1007/BF02861426. ISSN 0013-0001. http://link.springer.com/10.1007/BF02861426.
- ↑ Hillig, Karl W (1 October 2004). "A chemotaxonomic analysis of terpenoid variation in Cannabis" (in en). Biochemical Systematics and Ecology 32 (10): 875–891. doi:10.1016/j.bse.2004.04.004. https://linkinghub.elsevier.com/retrieve/pii/S0305197804001012.
- ↑ Aluko, R.E. (2017), "Hemp Seed (Cannabis sativa L.) Proteins" (in en), Sustainable Protein Sources (Elsevier): 121–132, doi:10.1016/b978-0-12-802778-3.00007-x, ISBN 978-0-12-802778-3, https://linkinghub.elsevier.com/retrieve/pii/B978012802778300007X
- ↑ Abel, Ernest L. (1980), "Cannabis in the Ancient World" (in en), Marihuana (Boston, MA: Springer US): 3–35, doi:10.1007/978-1-4899-2189-5_1, ISBN 978-1-4899-2191-8, http://link.springer.com/10.1007/978-1-4899-2189-5_1
- ↑ Bonini, Sara Anna; Premoli, Marika; Tambaro, Simone; Kumar, Amit; Maccarinelli, Giuseppina; Memo, Maurizio; Mastinu, Andrea (5 December 2018). "Cannabis sativa: A comprehensive ethnopharmacological review of a medicinal plant with a long history". Journal of Ethnopharmacology 227: 300–315. doi:10.1016/j.jep.2018.09.004. ISSN 1872-7573. PMID 30205181. https://pubmed.ncbi.nlm.nih.gov/30205181.
- ↑ Mediavilla, V; Leupin, M; Keller, A (1 January 2001). "Influence of the growth stage of industrial hemp on the yield formation in relation to certain fibre quality traits" (in en). Industrial Crops and Products 13 (1): 49–56. doi:10.1016/S0926-6690(00)00052-2. https://linkinghub.elsevier.com/retrieve/pii/S0926669000000522.
- ↑ Cosentino, Salvatore L.; Riggi, Ezio; Testa, Giorgio; Scordia, Danilo; Copani, Venera (1 October 2013). "Evaluation of European developed fibre hemp genotypes (Cannabis sativa L.) in semi-arid Mediterranean environment" (in en). Industrial Crops and Products 50: 312–324. doi:10.1016/j.indcrop.2013.07.059. https://linkinghub.elsevier.com/retrieve/pii/S0926669013003968.
- ↑ 19.0 19.1 Bewley-Taylor, D.; Blickman T.; Jelsma, M. (March 2014). "The Rise and Decline of Cannabis Prohibition: The History of Cannabis in the UN Drug Control System and Options for Reform". In Aronson, D. (PDF). Transnational Institute. https://www.tni.org/files/download/rise_and_decline_web.pdf.
- ↑ Gaoni, Y.; Mechoulam, R. (1 April 1964). "Isolation, Structure, and Partial Synthesis of an Active Constituent of Hashish" (in en). Journal of the American Chemical Society 86 (8): 1646–1647. doi:10.1021/ja01062a046. ISSN 0002-7863. https://pubs.acs.org/doi/abs/10.1021/ja01062a046.
- ↑ Šantavý, F. (1964). "Notes on the structure of cannabidiol compounds". Acta Universitatis Palackianae Olomucensis Facultatis Medicae 35: 5–9.
- ↑ Adams, Roger; Pease, D. C.; Cain, C. K.; Baker, B. R.; Clark, J. H.; Wolff, Hans; Wearn, R. B. (1 August 1940). "CONVERSION OF CANNABIDIOL TO A PRODUCT WITH MARIHUANA ACTIVITY. A TYPE REACTION FOR SYNTHESIS OF ANALOGOUS SUBSTANCES. CONVERSION OF CANNABIDIOL TO CANNABINOL" (in en). Journal of the American Chemical Society 62 (8): 2245–2246. doi:10.1021/ja01865a508. ISSN 0002-7863. https://pubs.acs.org/doi/abs/10.1021/ja01865a508.
- ↑ Devane, W. A.; Dysarz, F. A.; Johnson, M. R.; Melvin, L. S.; Howlett, A. C. (1 November 1988). "Determination and characterization of a cannabinoid receptor in rat brain". Molecular Pharmacology 34 (5): 605–613. ISSN 0026-895X. PMID 2848184. https://pubmed.ncbi.nlm.nih.gov/2848184.
- ↑ Devane, William A.; Hanuš, Lumir; Breuer, Aviva; Pertwee, Roger G.; Stevenson, Lesley A.; Griffin, Graeme; Gibson, Dan; Mandelbaum, Asher et al. (18 December 1992). "Isolation and Structure of a Brain Constituent That Binds to the Cannabinoid Receptor" (in en). Science 258 (5090): 1946–1949. doi:10.1126/science.1470919. ISSN 0036-8075. https://www.science.org/doi/10.1126/science.1470919.
- ↑ Simonsen, J. L.; Todd, A. R. (1942). "32. Cannabis indica. Part X. The essential oil from Egyptian hashish" (in en). Journal of the Chemical Society (Resumed): 188. doi:10.1039/jr9420000188. ISSN 0368-1769. http://xlink.rsc.org/?DOI=jr9420000188.
- ↑ 26.0 26.1 26.2 Hanuš, Lumír Ondřej; Hod, Yotam (10 August 2020). "Terpenes/Terpenoids in Cannabis: Are They Important?" (in en). Medical Cannabis and Cannabinoids 3 (1): 25–60. doi:10.1159/000509733. ISSN 2504-3889. PMC PMC8489319. PMID 34676339. https://www.karger.com/Article/FullText/509733.
- ↑ Ben-Shabat, Shimon; Fride, Ester; Sheskin, Tzviel; Tamiri, Tsippy; Rhee, Man-Hee; Vogel, Zvi; Bisogno, Tiziana; De Petrocellis, Luciano et al. (1 July 1998). "An entourage effect: inactive endogenous fatty acid glycerol esters enhance 2-arachidonoyl-glycerol cannabinoid activity" (in en). European Journal of Pharmacology 353 (1): 23–31. doi:10.1016/S0014-2999(98)00392-6. https://linkinghub.elsevier.com/retrieve/pii/S0014299998003926.
- ↑ 28.0 28.1 Russo, Ethan B (1 August 2011). "Taming THC: potential cannabis synergy and phytocannabinoid-terpenoid entourage effects: Phytocannabinoid-terpenoid entourage effects" (in en). British Journal of Pharmacology 163 (7): 1344–1364. doi:10.1111/j.1476-5381.2011.01238.x. PMC PMC3165946. PMID 21749363. https://onlinelibrary.wiley.com/doi/10.1111/j.1476-5381.2011.01238.x.
- ↑ Gallily, Ruth; Yekhtin, Zhannah; Hanuš, Lumír Ondřej (2018). "The Anti-Inflammatory Properties of Terpenoids from Cannabis". Cannabis and Cannabinoid Research 3 (1): 282–290. doi:10.1089/can.2018.0014. ISSN 2578-5125. PMC 6308289. PMID 30596146. https://pubmed.ncbi.nlm.nih.gov/30596146.
- ↑ Baron, Eric P. (1 July 2018). "Medicinal Properties of Cannabinoids, Terpenes, and Flavonoids in Cannabis, and Benefits in Migraine, Headache, and Pain: An Update on Current Evidence and Cannabis Science". Headache 58 (7): 1139–1186. doi:10.1111/head.13345. ISSN 1526-4610. PMID 30152161. https://pubmed.ncbi.nlm.nih.gov/30152161.
- ↑ Lewis, Mark A.; Russo, Ethan B.; Smith, Kevin M. (1 March 2018). "Pharmacological Foundations of Cannabis Chemovars". Planta Medica 84 (4): 225–233. doi:10.1055/s-0043-122240. ISSN 1439-0221. PMID 29161743. https://pubmed.ncbi.nlm.nih.gov/29161743.
- ↑ Horne, Matthew R.L. (2020), "Bast fibres" (in en), Handbook of Natural Fibres (Elsevier): 163–196, doi:10.1016/b978-0-12-818398-4.00007-4, ISBN 978-0-12-818398-4, https://linkinghub.elsevier.com/retrieve/pii/B9780128183984000074
- ↑ Réquilé, Samuel; Le Duigou, Antoine; Bourmaud, Alain; Baley, Christophe (1 November 2018). "Peeling experiments for hemp retting characterization targeting biocomposites" (in en). Industrial Crops and Products 123: 573–580. doi:10.1016/j.indcrop.2018.07.012. https://linkinghub.elsevier.com/retrieve/pii/S0926669018306125.
- ↑ Anderson, Loran C. (1 March 1980). "Leaf Variation among Cannabis Species from a Controlled Garden". Botanical Museum leaflets, Harvard University 28 (1): 61–69. doi:10.5962/p.168641. ISSN 0006-8098. https://www.biodiversitylibrary.org/part/168641.
- ↑ Schultes, Richard Evans; Klein, William M.; Plowman, Timothy; Lockwood, Tom E. (31 December 1975), Rubin, Vera, ed., "Cannabis: An Example of Taxonomic Neglect", Cannabis and Culture (DE GRUYTER MOUTON): 21–38, doi:10.1515/9783110812060.21, ISBN 978-90-279-7669-7, https://www.degruyter.com/document/doi/10.1515/9783110812060.21/html
- ↑ Cai, Changyong; Yu, Wang; Wang, Chaoyun; Liu, Lianglei; Li, Fenfang; Tan, Zhijian (1 August 2019). "Green extraction of cannabidiol from industrial hemp (Cannabis sativa L.) using deep eutectic solvents coupled with further enrichment and recovery by macroporous resin" (in en). Journal of Molecular Liquids 287: 110957. doi:10.1016/j.molliq.2019.110957. https://linkinghub.elsevier.com/retrieve/pii/S0167732218365899.
- ↑ Wiebelhaus, Nancy; Hamblin, D’Nisha; Kreitals, Natasha M.; Almirall, Jose R. (1 November 2016). "Differentiation of marijuana headspace volatiles from other plants and hemp products using capillary microextraction of volatiles (CMV) coupled to gas-chromatography–mass spectrometry (GC–MS)" (in en). Forensic Chemistry 2: 1–8. doi:10.1016/j.forc.2016.08.004. https://linkinghub.elsevier.com/retrieve/pii/S2468170916300285.
- ↑ Hammond, Charles T.; Mahlberg, Paul G. (1 September 1977). "MORPHOGENESIS OF CAPITATE GLANDULAR HAIRS OF CANNABIS SATIVA (CANNABACEAE)" (in en). American Journal of Botany 64 (8): 1023–1031. doi:10.1002/j.1537-2197.1977.tb11948.x. https://onlinelibrary.wiley.com/doi/10.1002/j.1537-2197.1977.tb11948.x.
- ↑ Potter, D. (March 2009). "The Propagation, Characterisation and Optimisation of Cannabis sativa L. as a Phytopharmaceutical" (PDF). King’s College London. https://extractionmagazine.com/wp-content/uploads/2018/06/THE-PROPAGATION-CHARACTERISATION-AND-OPTIMISATION-OF-CANNABIS-SATIVA-L-AS-A-PHYTOPHARMACEUTICAL.pdf.
- ↑ Livingston, Samuel J.; Quilichini, Teagen D.; Booth, Judith K.; Wong, Darren C. J.; Rensing, Kim H.; Laflamme-Yonkman, Jessica; Castellarin, Simone D.; Bohlmann, Joerg et al. (1 January 2020). "Cannabis glandular trichomes alter morphology and metabolite content during flower maturation". The Plant Journal: For Cell and Molecular Biology 101 (1): 37–56. doi:10.1111/tpj.14516. ISSN 1365-313X. PMID 31469934. https://pubmed.ncbi.nlm.nih.gov/31469934.
- ↑ Gupta, Asheesh Kumar; Jain, Anurekha; Roy, Partha; Singh, Ramandeep (2 October 2020). "Pharmacological Evaluation of Cannabis indica For Their Aphrodisiac Potential". International Journal of Ayurvedic Medicine 11 (3): 399–404. doi:10.47552/ijam.v11i3.1647. ISSN 0976-5921. https://www.ijam.co.in/index.php/ijam/article/view/1647.
- ↑ Ahmed, Aziez; Shapiro, Douglas; Su, Jennifer; Nelson, Lara P. (1 May 2021). "Vaping Cannabis Butane Hash Oil Leads to Severe Acute Respiratory Distress Syndrome-A Case of EVALI in a Teenager With Hypertrophic Cardiomyopathy". Journal of Intensive Care Medicine 36 (5): 617–621. doi:10.1177/0885066620941004. ISSN 1525-1489. PMID 32686568. https://pubmed.ncbi.nlm.nih.gov/32686568.
- ↑ Stephens, Danica; Patel, Jinal K.; Angelo, Debra; Frunzi, Johnathan (18 February 2020). "Cannabis Butane Hash Oil Dabbing Induced Lung Injury Mimicking Atypical Pneumonia". Cureus 12 (2): e7033. doi:10.7759/cureus.7033. ISSN 2168-8184. PMC 7082782. PMID 32211266. https://pubmed.ncbi.nlm.nih.gov/32211266.
- ↑ Schwachtje, Jens; Baldwin, Ian T. (1 March 2008). "Why does herbivore attack reconfigure primary metabolism?". Plant Physiology 146 (3): 845–851. doi:10.1104/pp.107.112490. ISSN 0032-0889. PMC 2259057. PMID 18316639. https://pubmed.ncbi.nlm.nih.gov/18316639.
- ↑ Sommano, Sarana (30 November 2013), P. M, Visakh; Iturriaga, Laura B.; Ribotta, Pablo Daniel, eds., "Effect of Food Processing on Bioactive Compounds" (in en), Advances in Food Science and Nutrition (Hoboken, NJ, USA: John Wiley & Sons, Inc.): 361–390, doi:10.1002/9781118865606.ch11, ISBN 978-1-118-86560-6, https://onlinelibrary.wiley.com/doi/10.1002/9781118865606.ch11
- ↑ Sommano, Sarana; Caffin, Nola; Kerven, Graham (18 August 2013). "Screening for Antioxidant Activity, Phenolic Content, and Flavonoids from Australian Native Food Plants" (in en). International Journal of Food Properties 16 (6): 1394–1406. doi:10.1080/10942912.2011.580485. ISSN 1094-2912. http://www.tandfonline.com/doi/abs/10.1080/10942912.2011.580485.
- ↑ Sunanta, Piyachat; Chung, Hsiao‐Hang; Kunasakdakul, Kaewalin; Ruksiriwanich, Warintorn; Jantrawut, Pensak; Hongsibsong, Surat; Sommano, Sarana Rose (1 August 2020). "Genomic relationship and physiochemical properties among raw materials used for Thai black garlic processing" (in en). Food Science & Nutrition 8 (8): 4534–4545. doi:10.1002/fsn3.1762. ISSN 2048-7177. PMC PMC7455981. PMID 32884733. https://onlinelibrary.wiley.com/doi/10.1002/fsn3.1762.
- ↑ Wisetkomolmat, Jiratchaya; Suppakittpaisarn, Pongsakorn; Sommano, Sarana Rose (3 January 2019). "Detergent Plants of Northern Thailand: Potential Sources of Natural Saponins" (in en). Resources 8 (1): 10. doi:10.3390/resources8010010. ISSN 2079-9276. https://www.mdpi.com/2079-9276/8/1/10.
- ↑ Tangpao, Tibet; Chung, Hsiao-Hang; Sommano, Sarana (24 October 2018). "Aromatic Profiles of Essential Oils from Five Commonly Used Thai Basils" (in en). Foods 7 (11): 175. doi:10.3390/foods7110175. ISSN 2304-8158. PMC PMC6262289. PMID 30352978. http://www.mdpi.com/2304-8158/7/11/175.
- ↑ Ross, Samir A.; ElSohly, Mahmoud A. (1 January 1996). "The Volatile Oil Composition of Fresh and Air-Dried Buds of Cannabis sativa" (in en). Journal of Natural Products 59 (1): 49–51. doi:10.1021/np960004a. ISSN 0163-3864. https://pubs.acs.org/doi/10.1021/np960004a.
- ↑ Turner, C. E.; Elsohly, M. A.; Boeren, E. G. (1 March 1980). "Constituents of Cannabis sativa L. XVII. A review of the natural constituents". Journal of Natural Products 43 (2): 169–234. doi:10.1021/np50008a001. ISSN 0163-3864. PMID 6991645. https://pubmed.ncbi.nlm.nih.gov/6991645.
- ↑ Wanas, Amira S.; Radwan, Mohamed M.; Chandra, Suman; Lata, Hemant; Mehmedic, Zlatko; Ali, Abbas; Baser, Khc; Demirci, Betul et al. (1 May 2020). "Chemical Composition of Volatile Oils of Fresh and Air-Dried Buds of Cannabis c hemovars, Their Insecticidal and Repellent Activities" (in en). Natural Product Communications 15 (5): 1934578X2092672. doi:10.1177/1934578X20926729. ISSN 1934-578X. http://journals.sagepub.com/doi/10.1177/1934578X20926729.
- ↑ Rice, Somchai; Koziel, Jacek A. (10 December 2015). Glendinning, John I.. ed. "Characterizing the Smell of Marijuana by Odor Impact of Volatile Compounds: An Application of Simultaneous Chemical and Sensory Analysis" (in en). PLOS ONE 10 (12): e0144160. doi:10.1371/journal.pone.0144160. ISSN 1932-6203. PMC PMC4684335. PMID 26657499. https://dx.plos.org/10.1371/journal.pone.0144160.
- ↑ Ternelli, Marco; Brighenti, Virginia; Anceschi, Lisa; Poto, Massimiliano; Bertelli, Davide; Licata, Manuela; Pellati, Federica (15 July 2020). "Innovative methods for the preparation of medical Cannabis oils with a high content of both cannabinoids and terpenes". Journal of Pharmaceutical and Biomedical Analysis 186: 113296. doi:10.1016/j.jpba.2020.113296. ISSN 1873-264X. PMID 32334134. https://pubmed.ncbi.nlm.nih.gov/32334134.
- ↑ Wang, Chi-Tsan; Ashworth, Kirsti; Wiedinmyer, Christine; Ortega, John; Harley, Peter C.; Rasool, Quazi Z.; Vizuete, William (1 July 2020). "Ambient measurements of monoterpenes near Cannabis cultivation facilities in Denver, Colorado" (in en). Atmospheric Environment 232: 117510. doi:10.1016/j.atmosenv.2020.117510. https://linkinghub.elsevier.com/retrieve/pii/S1352231020302478.
- ↑ Abdollahi, Mahnaz; Sefidkon, Fatemeh; Calagari, Mohsen; Mousavi, Amir; Mahomoodally, M. Fawzi (1 November 2020). "Impact of four hemp (Cannabis sativa L.) varieties and stage of plant growth on yield and composition of essential oils" (in en). Industrial Crops and Products 155: 112793. doi:10.1016/j.indcrop.2020.112793. https://linkinghub.elsevier.com/retrieve/pii/S092666902030710X.
Notes
This presentation is faithful to the original, with only a few minor changes to presentation. Some grammar and punctuation was cleaned up to improve readability. In some cases important information was missing from the references, and that information was added.