Journal:Recent advances in electrochemical sensor technologies for THC detection—A narrative review
| Full article title | Recent advances in electrochemical sensor technologies for THC detection—A narrative review |
|---|---|
| Journal | Journal of Cannabis Research |
| Author(s) | Amini, Kaveh; Sepehrifard, Ali; Valinasabpouri, Ali; Safruk, Jennifer; Angelone, Davide; de Campos Lourenco, Tiago |
| Author affiliation(s) | Selective Lab, Inc. |
| Primary contact | Email: kamini at selectivelab dot com |
| Year published | 2022 |
| Volume and issue | 4 |
| Article # | 12 |
| DOI | 10.1186/s42238-022-00122-3 |
| ISSN | 2522-5782 |
| Distribution license | Creative Commons Attribution 4.0 International |
| Website | https://jcannabisresearch.biomedcentral.com/articles/10.1186/s42238-022-00122-3 |
| Download | https://jcannabisresearch.biomedcentral.com/track/pdf/10.1186/s42238-022-00122-3.pdf (PDF) |
Abstract
Background: delta-9-tetrahydrocannabinol (Δ9-THC or simply THC) is the main psychoactive component and one of the most important medicinal compounds in cannabis. Whether in human body fluids and breath or in laboratory and field samples, rapid and easy detection of THC is crucial. It provides insights into the impact of THC on the human organism, as well as its medicinal benefits. It also guides cannabis growers in determining different stages of the growth of the plant in the field, and eventually it helps scientists in the laboratory to assure the quality of the products and determine their potency or better understand product development procedures. The significance of fast THC detection in forensic analysis also cannot be overlooked. Electrochemical sensor technologies show promise in fulfilling the need for fast, easy, and low-cost detection of THC.
Method: In this work, we review the recent advances in sensor technologies developed for the purpose of fast and accurate THC detection. Research performed mostly in the past decade, and that detecting THC directly without any derivatization, was the main target of this review. The scope of this narrative review was reports on detecting THC in synthetic samples and plants as well as oral fluid.
Results: Electrochemical sensor technologies are sensitive enough and have the potential for fast, easy, and low-cost detection of THC for roadside testing, THC trending in growing cannabis plants, THC product development, and formulation for medical purposes, etc. They can also provide an alternative for costly chromatography and mass spectrometry-based methods.
Conclusion: The main challenges facing electrochemical sensors, however, are nonspecific interaction and the interference of compounds and species from the matrix. Special requirement for storing sensors modified with antibodies or proteins is another challenge in this field. Preparing long-lasting and reusable sensors is a field worthy of attention.
Keywords: Δ9-tetrahydrocannabinol, THC, sensor, cannabis, electrochemical detection
Introduction
In light of the legalization of medicinal and recreational cannabis in Canada and many states in the United Stats, as well as other countries around the world, a great deal of attention has been given to the research in different areas of cannabis chemistry.[1] Additionally, a growing body of evidence for the therapeutic effects of cannabis has turned cannabis research into a hot topic.[2][3] Over 60 unique cannabinoids have been identified in the Cannabis plant.[4] Out of these cannabinoids, delta-9-tetrahydrocannabinol (Δ9-THC or simply THC), cannabidiol (CBD), and cannabinol (CBN) are the most significant ones.[5] THC is responsible for the psychoactive property of cannabis and causes euphoria, drowsiness, hallucinations, and temporal distortions.[6] CBD, another significant cannabinoid, on the other hand is not psychoactive; however, it has neuroprotective, sedating, anti-inflammatory, and analgesic impacts.[7][8][9][10]
However, the legalization of cannabis has also raised concerns about driving under the influence of THC. An increase in the number of cases of THC-impaired driving has been reported in regions of the world where cannabis has been legalized.[11][12][13] This has led to questions and doubts about how to best approach testing for potential THC levels in drivers.[14] Electrochemical sensors may make for one possible approach to this problem, as well for other cannabis testing applications.
In this work, we critically review the recent developments and advances in THC detection through sensor technologies, as well as the potential presented for different applications, from forensic and law enforcement to quality control and product development. First, a brief background on electrochemical sensors and their potential in THC analysis is given. Then sensors are discussed in two major categories: (a) early applications in synthetic solutions and plant analysis and (b) applications in oral fluid analysis. Sensors focused on THC detection in breath, which are thoroughly reviewed by Ramzy and Priefer[15], have not been included in this review.
Background on electrochemical sensors for THC analysis
Electrochemical sensors provide a highly sensitive tool for the analysis of THC, and they also have advantages such as easy miniaturization and usability in turbid matrices. In these sensors, the current or potential change, which occurs as a result of the interactions or reactions at the interface between the sensor surface and the samples solution, is measured. On the basis of the electrochemical technique used for detection, these sensors can be broadly categorized as potentiometric, impedimetric, voltammetric, and amperometric.[16][17] In potentiometric sensors, measurements are based on the development of electrochemical potential in proportion to the activity of the analyte.[16][18] In impedimetric sensors, electrochemical impedance spectroscopy (EIS) is employed as the detection technique. In these sensors, a low voltage sinusoidal potential is applied at different frequencies to the sensor and the impedance is measured as a function of frequencies using the resulting current. The interaction between the analyte and a biorecognition element immobilized on the sensor surface will cause changes in the impedance. The results will be interpreted in terms of an equivalent circuit.[16][19] (Another major advantage of impedance-based sensors is being label-free.[16][20]) Voltammetric sensors employ electrochemical techniques such as cyclic voltammetry, square wave voltammetry, and differential pulse voltammetry, whereas in the case of amperometric sensors, the changes in current are followed.[16][21]
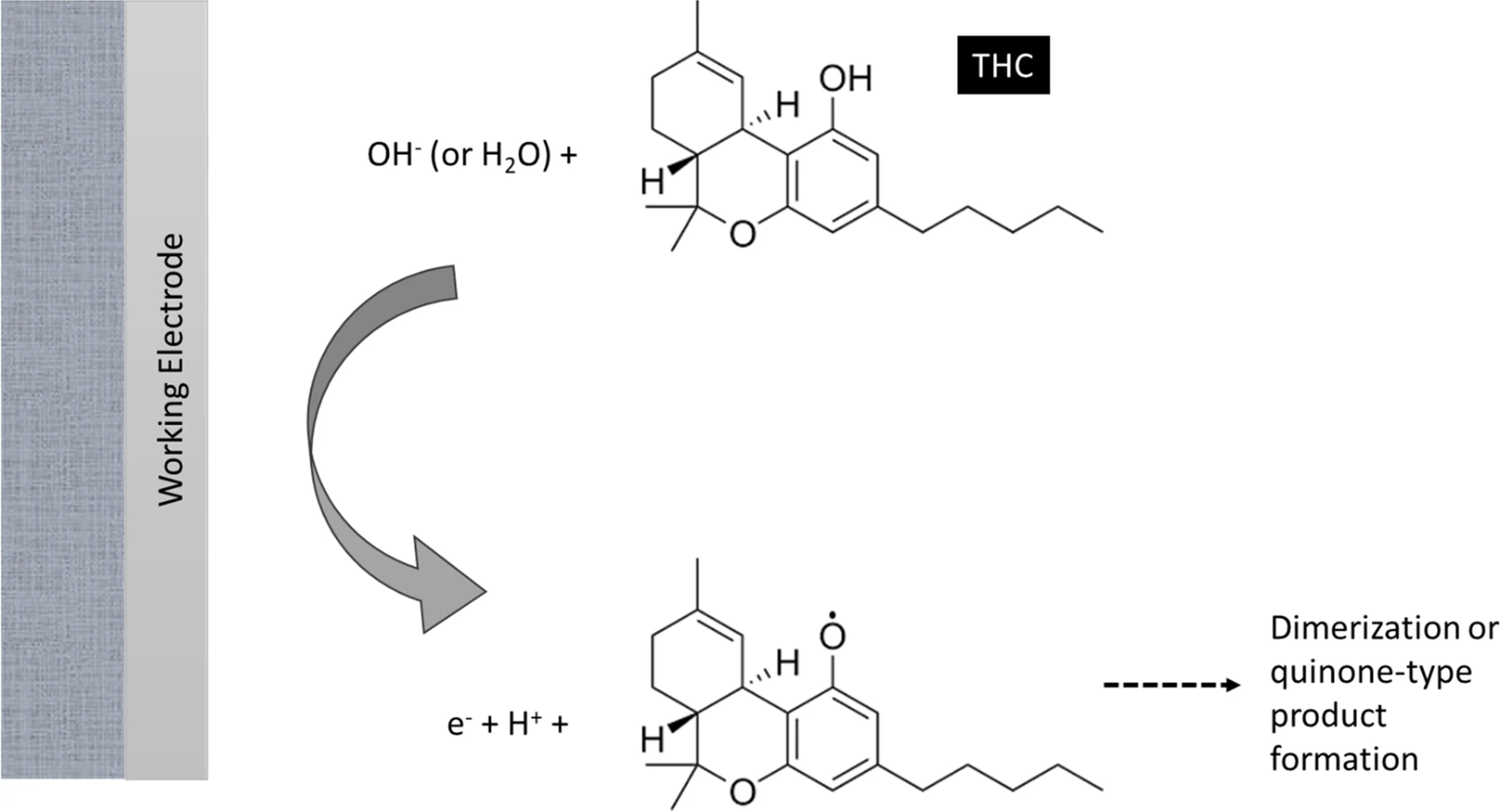
Figure 1 illustrates the mechanism under which electrochemical sensors operate towards the detection of THC, according to Renaud-Young et al.[22]
|
The standard THC detection method includes costly, complex, and time-consuming steps of obtaining a clinical specimen or botanical sample and analyzing it using chromatography with mass spectrometry detection. This method obviously would be cumbersome when it comes to roadside testing or testing plant material directly in the field. The limitations of standard THC detection methods for these testing purposes have motivated scientists to develop portable, non-invasive, fast, and low-cost sensor technologies for on-site THC screening and testing.[23] Sensors capable of detecting and quantifying THC and other cannabinoids can also be very effective in other areas such as monitoring different stages of plant growth, performing quality control on cannabis products, and while developing products from cannabis. Also, ease-of-use—alongside rapid detection—for these sensors makes them ideal to be used by dispensaries or consumers to evaluate the THC levels or THC-CBD ratio of the products they acquire.[24] In comparison to other portable methods, sensor-based technologies have the potential to reduce analysis time and cost, as well as improve sensitivity through the process of miniaturization[16][25][26][27], offering a novel and alternative approach to testing cannabinoids like THC for different purposes.
Development of early sensors and trials in synthetic solutions and plant extracts
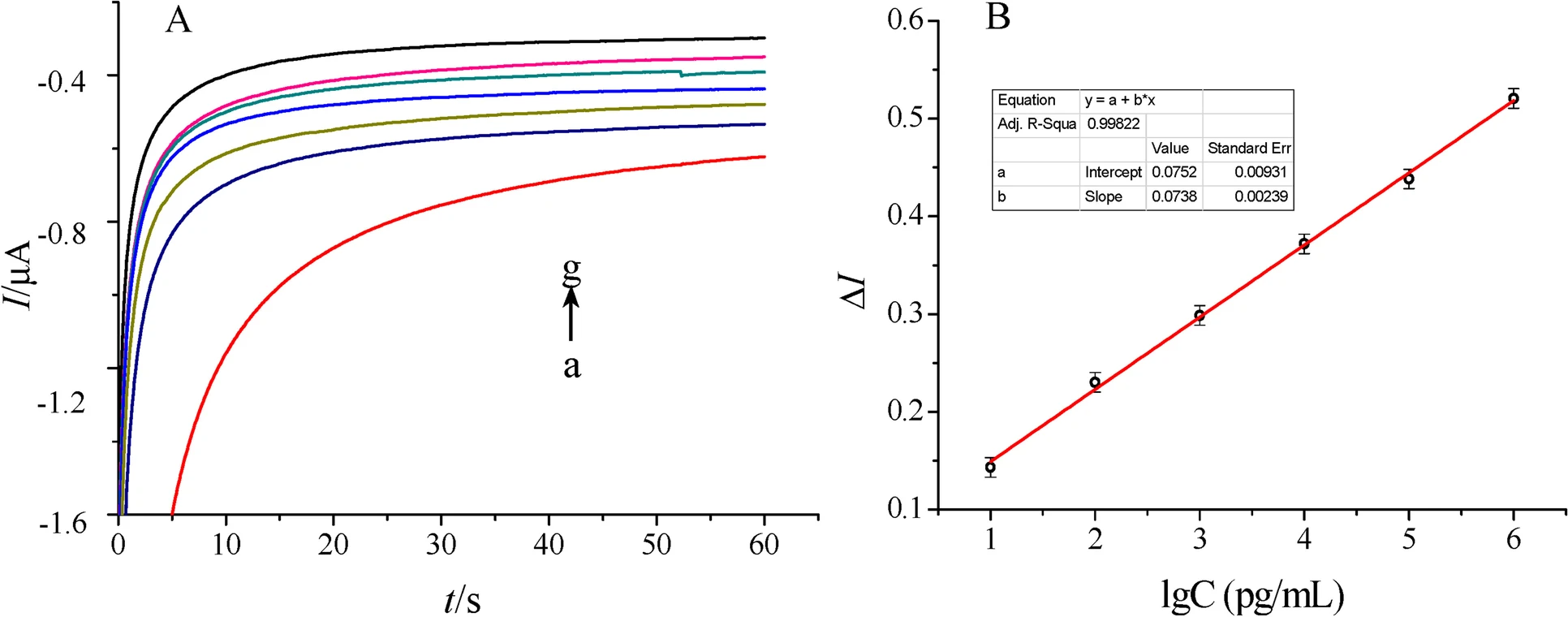
The first attempt towards the development of a THC sensor has been reported by Lu et al.[28] To develop this sensor, a THC antibody derived from Balb/c mice as the bio-recognition element was immobilized on a novel double-layer gold nanoparticle electrode. Also, an electrochemical biosensing signal amplification system with gold nanoparticle-thionine-chitosan absorbing horseradish peroxidase (HRP) was used to enhance the number of immobilized antibodies and thus the electrochemical signal. Developed biosensors were employed to determine THC in phosphate buffer saline (PBS) using the amperometric I-t curve method. The dynamic linear range of the calibration curve made by plotting response current versus THC concentration was from 0.01~103 ng/mL, with a correlation coefficient of 0.9986. The lowest limit of detection (LOD) for THC also was reported to be 3.3 pg/mL (S/N = 3), with good sensitivity and reproducibility. Current response curves for determination of THC and the calibration curve constructed in the work of Lu et al. [28] are illustrated in Fig. 2.
|
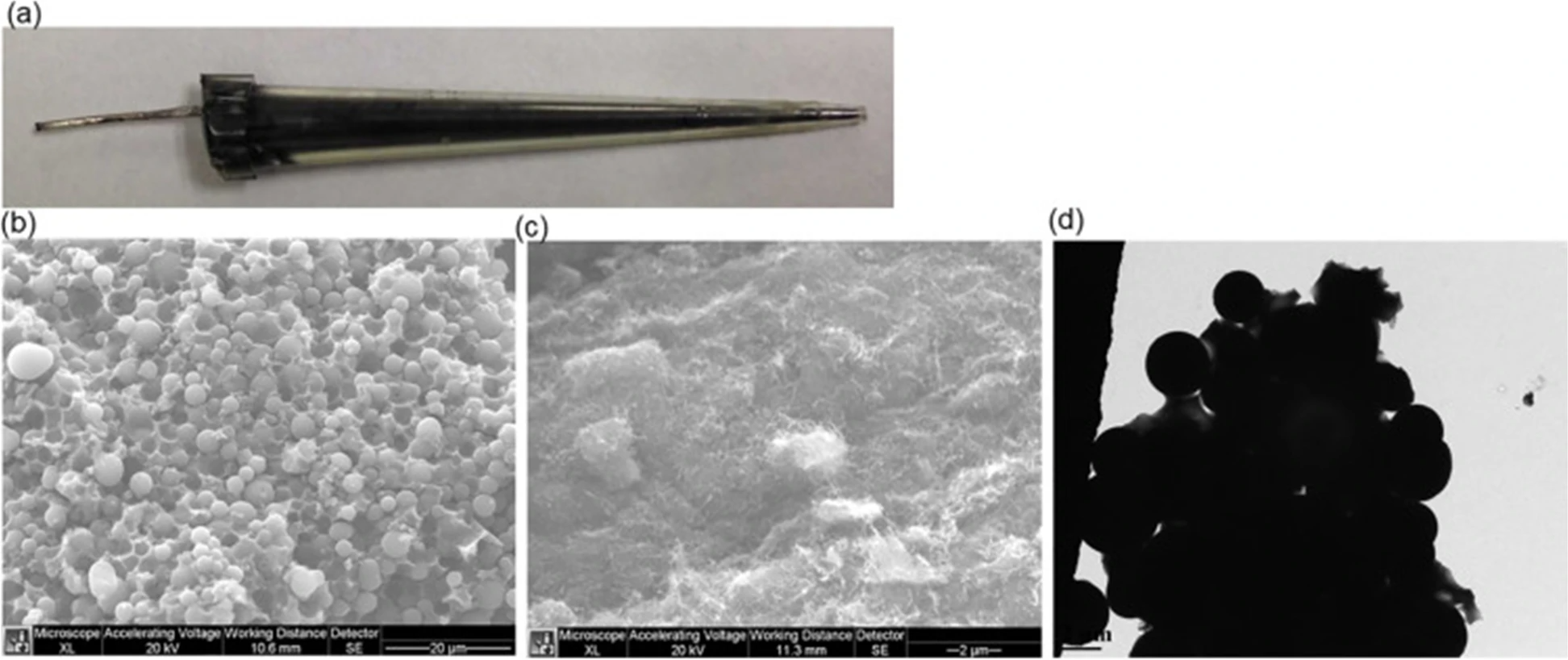
More recently, Zhang et al.[29] employed carbon nanotubes (CNT) or carbon beads and poly(methyl acrylic acid-co-ethylene glycol dimethacrylate) (poly(MAA-Co-EGDMA)) with molecularly imprinting technology in micropipette tubes to develop THC sensors. Molecularly imprinted polymers (MIPs) are synthetic receptors which can selectively bind to their target molecules and, therefore, can be used as recognition elements in sensors as a replacement for relatively unstable bio-recognition elements such as enzymes and antibodies. Zhang et al. emphasized the importance of utilizing carbon materials in sensors, which results in a high sensitivity because of the high surface area, as well as appropriate MIPs technique to create THC molecular cavities on the surface of carbon micro-beads and carbon nanotubes (CNT) packed in plastic micropipette tips. These THC MIPs were synthesized by copolymerization of methacrylic acid (MAA) and ethylene glycol dimethacrylate (EDGMA) in presence of THC initiated with 4,4′-azobis (4-cyanovaleric Acid) (AIBN), and then THC was removed to generate THC molecular cavities for specific binding of THC. Optical and microscopic images of their developed sensors are shown in Fig. 3. The developed sensors showed high selectivity towards THC over caffeine and acetaminophen. The detection limit of THC using CNT-MIP sensors was determined to be 0.18 ± 0.02 ng/mL, which is significantly improved in comparison to the detection limit using nonimprinted polymers (NIP), which was 12.5 ± 0.5 ng/mL.[29]
|
Another work employing MIPs was introduced by Canfarotta et al.[30] In their research, a manufacturing-friendly protocol for integration of MIP nanoparticles (nanoMIPs) with a (label-free) capacitive sensor was developed. The two templates for which the nanoMIPs were produced included THC as a small molecule and trypsin as a protein. Using this sensor, determining THC was demonstrated to be possible at physiological concentrations. Another advantage of these sensors was the possibility of using them for detection and quantification of other biomolecules by varying the nanoMIPs. These nanoMIPs can then be virtually produced against any target.[30]
Sensors developed for THC detection in oral fluid specimens
Oral fluid or saliva analysis for roadside drug testing is a great substitute for routine blood or urine tests due to being particularly non-invasive while also removing the need for inconvenient observations during sample collection for urine analysis. These tests are normally performed as a screening step. Goodwin et al.[31] reported on a sensor developed by modification of micro-sized graphite powder by abrasive immobilization of 4-amino-2,6-diphenylphenol onto a basal plane pyrolytic graphite electrode. This sensor was then used for indirect detection of THC in oral fluid. The mechanism under which this sensor operates was described such that by addition of THC, the reduction wave—which corresponds to the electrochemical reduction of quinoneimine (QI) back to aminophenol (AP)—reduces in magnitude due to the reaction between QI and THC, and this, in turn, provides a useful analytical signal. This technique was indicated as having potential because it does not involve direct oxidation of THC, which can cause electrode passivation. Goodwin et al. also reported that such a device has the potential to detect THC using screen-printed electrodes and similar approaches. The construction of the sensor was done through machining pyrolytic graphite discs into a 4.9 mm diameter. The counter electrode was a platinum wire, and the measurements were performed against a saturated calomel electrode. The linear dynamic range of the sensor was reported to be from 1.25 to 25 μM, with an LOD of 1 μM.[31]
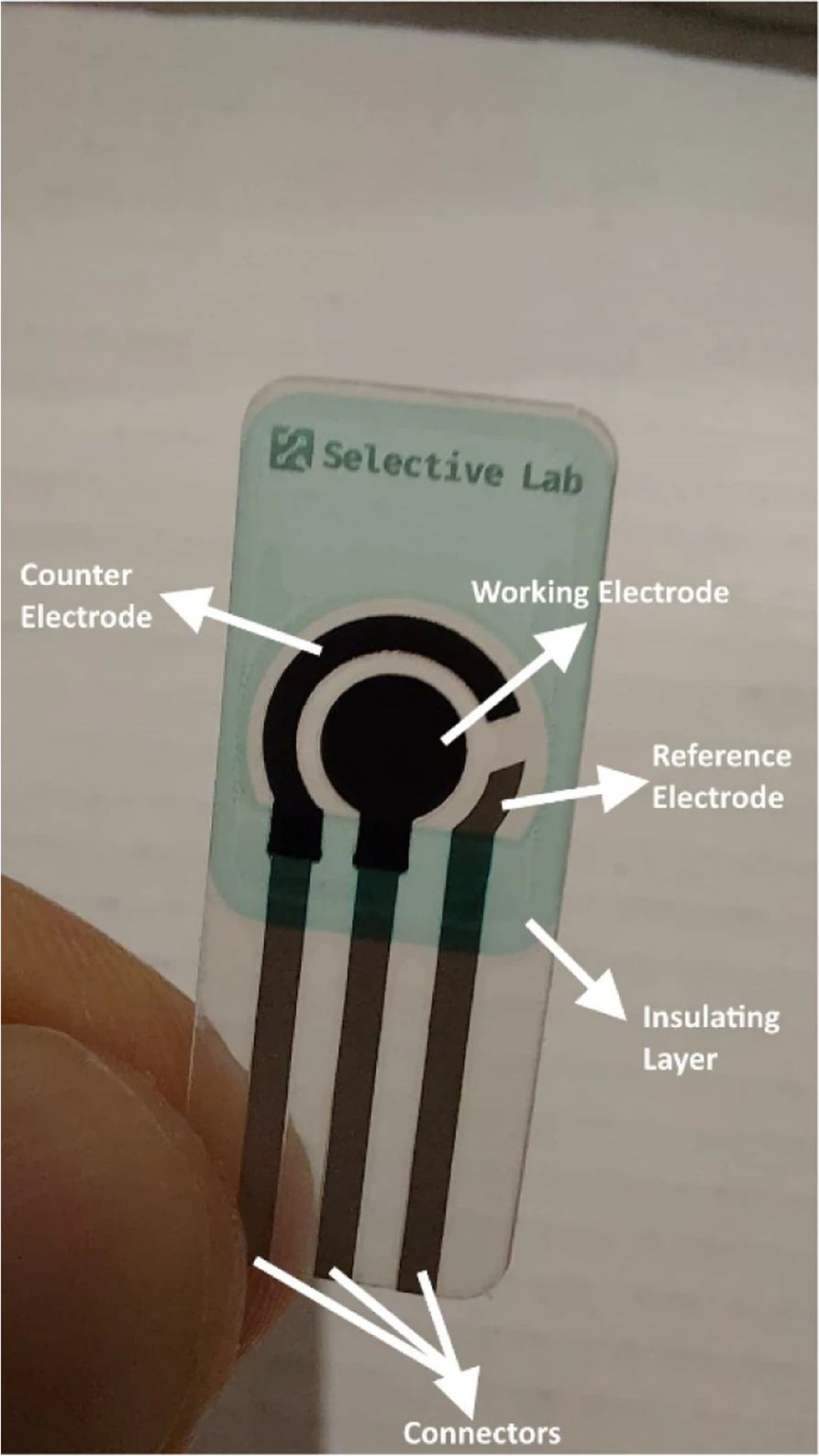
Modern screen-printed electrodes are produced by using different kinds of inks on various types of plastic or ceramic substrates. A wide variety of these screen-printed electrodes are commercially available. The versatility of screen-printed electrodes is found in the wide range of possibilities for the modification of the electrodes. The composition of the inks can be modified by addition of different substances such as metals, enzymes, polymers, complexing agents, etc. It is also possible to modify the electrodes by depositing different substances on the surface of the electrodes such as metal films, polymers, enzymes, etc.[32] Figure 4 illustrates a screen-printed electrode and its different parts.
|
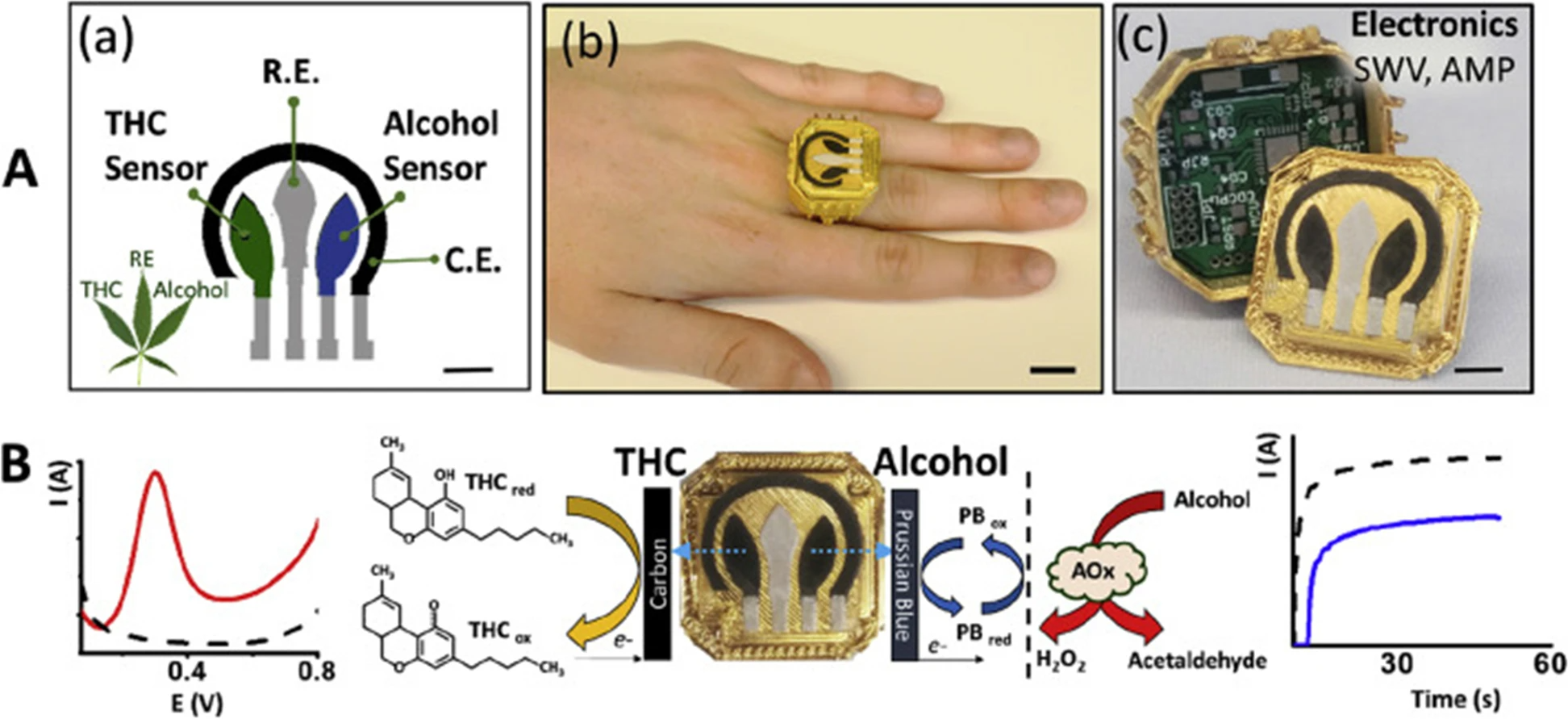
In another interesting report, Mishra et al.[33] presented work on a wearable electrochemical sensor for the simultaneous direct, decentralized detection of THC and alcohol in oral fluids. This sensor was designed in the form of a ring and includes a square-wave voltammetric THC sensor and an amperometric enzymatic alcohol sensor on the ring cap, with the wireless electronics embedded within the ring case. The disposable sensing electrode ring cap was designed so that it allows for fast replacement through alignment with the spring-loaded pins, mounted on the printed circuit board (PCB), with the current collectors of the sensing electrodes, after each oral fluids test. The printed sensor for dual-analyte detection (ring cover) was composed of a multi-wall carbon nanotube/carbon electrode for THC detection and a Prussian-blue transducer, coated with an alcohol oxidase/chitosan reagent layer, for alcohol detection. This structure makes it possible for THC and alcohol to be detected simultaneously in the same diluted oral fluids specimen in three minutes without any interference from the matrix and also no cross-talk among sensors. Figure 5 illustrates the ring sensor developed by Mishra et al. The detection limits were indicted to be 0.5 μM for THC and 0.2 mM for alcohol. This wearable THC/alcohol sensor was found to be promising for both roadside drug testing and for a drivers’ self-assessment before driving.[33]
|
In another work, Nissim and Compton[34] introduced an optimized carbon paste electrode, made from graphite powder and mineral oil, for sensitive detection of THC in both aqueous solutions of pH 10.0 and in synthetic oral fluids. Absorptive stripping voltammetry was utilized as the detection technique. A copper rod with a radius of 1.97 mm running through a Teflon rod was used to fabricate the carbon paste electrode. The copper rod embedded in the Teflon rod was adjusted so that it left a 1.00 mm-deep cavity at the edge. Two sorts of pastes were used, which were made by mixing graphite powder with either dioctyl phthalate or mineral oil. 1.4 mL dioctyl phthalate and 4.26 g graphite powder were mixed to prepare the graphite/dioctyl phthalate paste. The same ratio also was used to prepare the graphite/mineral oil paste. The surface of the carbon paste electrode was renewed between each scan by packing it with fresh paste. Practical limits of detection for THC using this sensor were reported to be 0.50 μM and 0.10 μM in stationary and stirred aqueous borate buffer solutions, respectively, whereas the theoretical limits of detections were calculated to be 0.48 nM and 0.41 nM for stationary and stirred THC aqueous borate buffer solutions, respectively. This sensor was capable of detecting THC concentrations as low as 0.50 μM in synthetic oral fluids solutions. The sensor had sensitivities of 0.12 μA μM−1, 0.84 μA μM−1, and 0.067 μA μM−1 for the stationary buffer, the stirred buffer, and the oral fluids matrix, respectively.[34]
Wanklyn et al.[35] reported development of a screen-printed carbon electrode for N-(4-amino-3-methoxyphenyl)-methane sulfonamide-mediated detection of THC in oral fluids. The sensor was prepared by placing a dried reagent overlayer containing mediator, buffer, salt, and surfactant over the electrodes. When applying the sample to one end of the membrane, the sample would go through the overlayer and wet the reagents and the electrode surfaces and thus dissolve the mediator. The mediator would galvanostatically get oxidized and react with THC to form an electrochemically active adduct, which would be in turn detected by chronoamperometric reduction. The developed sensor was used to detect THC spiked in undiluted oral fluids at 25–50 ng/mL, with a response time of 30 seconds. A trial of these sensors on the oral fluid specimens from four cannabis smokers showed a sensitivity of 28%, specificity of 99%, and accuracy of 52%. The sensitivity of this sensor was indicated to be lower than the acceptable criteria.[35]
In 2019, Stevenson et al.[36] introduced an impedance-based sensor utilizing affinity biosensing to detect THC in oral fluid through its chemical reaction with a specific antibody. The detection limit for this sensor was indicated to be 100 pg/ml, and the dynamic linear range was 100 pg/ml–100 ng/ml in human oral fluid. This sensor proved to have a rapid detection time, i.e., less than one minute. In this sensor technology, non-faradaic electrochemical impedance spectroscopy was used to detect the presence of BSA-THC hapten in human oral fluid. The affinity biosensor was shown to detect the biomarker through a recognition element that specifically bound to the biomarker of interest. The chemical reaction between the biomarker and the recognition element was then converted into an electrical signal correlated to the concentration of the biomarker. This electrochemical biosensor was capable of streamlining the testing process, removing the need for sample preparation and reducing the analysis time.[36]
Conclusions and future perspectives drawn from these works
There is a great amount of focus by researchers around the world on the development of a hand-held, fast, and user-friendly device for the detection of THC for different applications of roadside drug testing, cannabis product quality control, and cannabis crop evaluation. The recent research works described here demonstrate an enormous potential for the application of electrochemical sensors for this purpose. The possibility of miniaturization and different modifications that allow for better sensitivity and selectivity, as well as lower costs and fast response, are only a few advantages of electrochemical sensors. In fact, most of these described sensors have been proven to be sensitive enough for trace THC detection found in oral fluids after cannabis consumption. LOD can also be sufficient; among all the sensors reviewed in this article, the sensor developed by Lu et al.[28] showed the lowest LOD under the stated research conditions.
The main challenges associated with these sensors, however, are nonspecific interaction and the interference of compounds and species from the heavy plant matrix or oral fluids. Also, sensors modified with antibodies or other proteins require special storage conditions, such as being refrigerated at certain temperatures. The lifetime of the bio-recognition elements used in these sensors is also limited. Development of stable and long-lasting sensors with a higher selectivity and minimum nonspecific interactions and matrix interference is now the major problem that the scientific and research community has to solve.
Acknowledgements
Author contributions
All authors contributed to the design of the review, had access to the data, and were involved in collecting the information and participated in drafting, revising, and approving the manuscript.
Competing interests
All authors are beneficiaries of, and receive financial compensation from, Selective Lab Inc., which develops sensors for THC (https://selectivelab.com/pages/about).
Selective Lab Inc. had no role in the design and conduct of the experiments or the analysis of the data reviewed in this manuscript.
References
- ↑ Crean, Rebecca D.; Crane, Natania A.; Mason, Barbara J. (1 March 2011). "An Evidence-Based Review of Acute and Long-Term Effects of Cannabis Use on Executive Cognitive Functions" (in en). Journal of Addiction Medicine 5 (1): 1–8. doi:10.1097/ADM.0b013e31820c23fa. ISSN 1932-0620. PMC PMC3037578. PMID 21321675. https://journals.lww.com/01271255-201103000-00001.
- ↑ Hill, Kevin P. (23 June 2015). "Medical Marijuana for Treatment of Chronic Pain and Other Medical and Psychiatric Problems". JAMA 313 (24): 2474. doi:10.1001/jama.2015.6199. ISSN 0098-7484. http://dx.doi.org/10.1001/jama.2015.6199.
- ↑ Hoffmann, Diane E.; Weber, Ellen (22 April 2010). "Medical Marijuana and the Law". New England Journal of Medicine 362 (16): 1453–1457. doi:10.1056/NEJMp1000695. ISSN 0028-4793. PMID 20410512. https://doi.org/10.1056/NEJMp1000695.
- ↑ Vemuri, V Kiran; Makriyannis, A (16 April 2015). "Medicinal chemistry of cannabinoids". Clinical Pharmacology & Therapeutics 97 (6): 553–558. doi:10.1002/cpt.115. ISSN 0009-9236. PMC PMC4918805. PMID 25801236. http://dx.doi.org/10.1002/cpt.115.
- ↑ Grotenhermen, Franjo (2003). "Pharmacokinetics and Pharmacodynamics of Cannabinoids:" (in en). Clinical Pharmacokinetics 42 (4): 327–360. doi:10.2165/00003088-200342040-00003. ISSN 0312-5963. http://link.springer.com/10.2165/00003088-200342040-00003.
- ↑ Ashton, C. Heather (2001/02). "Pharmacology and effects of cannabis: A brief review" (in en). The British Journal of Psychiatry 178 (2): 101–106. doi:10.1192/bjp.178.2.101. ISSN 0007-1250. https://www.cambridge.org/core/journals/the-british-journal-of-psychiatry/article/pharmacology-and-effects-of-cannabis-a-brief-review/82B02735F420CB287DCC80843FC34AE1.
- ↑ Chakravarti, Bandana; Ravi, Janani; Ganju, Ramesh K. (17 July 2014). "Cannabinoids as therapeutic agents in cancer: current status and future implications" (in en). Oncotarget 5 (15): 5852–5872. doi:10.18632/oncotarget.2233. ISSN 1949-2553. PMC PMC4171598. PMID 25115386. https://www.oncotarget.com/article/2233/text/.
- ↑ Hill, Kevin P. (10 September 2019). "Medical Use of Cannabis in 2019". JAMA 322 (10): 974–975. doi:10.1001/jama.2019.11868. ISSN 0098-7484. https://doi.org/10.1001/jama.2019.11868.
- ↑ Klimuntowski, Martin; Alam, Maksud M.; Singh, Gurmit; Howlader, Matiar M. R. (27 February 2020). "Electrochemical Sensing of Cannabinoids in Biofluids: A Noninvasive Tool for Drug Detection". ACS Sensors 5 (3): 620–636. doi:10.1021/acssensors.9b02390. ISSN 2379-3694. http://dx.doi.org/10.1021/acssensors.9b02390.
- ↑ Mechoulam, Raphael; Peters, Maximilian; Murillo-Rodriguez, Eric; Hanuš, Lumír O. (1 August 2007). "Cannabidiol – Recent Advances". Chemistry & Biodiversity 4 (8): 1678–1692. doi:10.1002/cbdv.200790147. ISSN 1612-1872. http://dx.doi.org/10.1002/cbdv.200790147.
- ↑ Kalant, Harold (2001). "Medicinal Use of Cannabis: History and Current Status" (in en). Pain Research and Management. doi:10.1155/2001/469629. https://www.hindawi.com/journals/prm/2001/469629/. Retrieved 2022-05-30.
- ↑ Zuardi, Antonio Waldo (1 June 2006). "History of cannabis as a medicine: a review". Revista Brasileira de Psiquiatria 28 (2): 153–157. doi:10.1590/S1516-44462006000200015. ISSN 1516-4446. http://www.scielo.br/scielo.php?script=sci_arttext&pid=S1516-44462006000200015&lng=en&tlng=en.
- ↑ Kim, S.Y.; Kim, J.Y.; Kwon, W. et al. (2013). "Method development for simultaneous determination of amphetamine type stimulants and cannabinoids in urine using GC–MS" (in en). Microchemical Journal 110: 326–333. doi:10.1016/j.microc.2013.04.004. ISSN 0026-265X.
- ↑ Pearlson, Godfrey D.; Stevens, Michael C.; D'Souza, Deepak Cyril (24 September 2021). "Cannabis and Driving". Frontiers in Psychiatry 12: 689444. doi:10.3389/fpsyt.2021.689444. ISSN 1664-0640. PMC PMC8499672. PMID 34630173. https://www.frontiersin.org/articles/10.3389/fpsyt.2021.689444/full.
- ↑ Ramzy, V.; Priefer, R. (2021). "THC detection in the breath". Talanta 222: 121528. doi:10.1016/j.talanta.2020.121528. ISSN 0039-9140.
- ↑ 16.0 16.1 16.2 16.3 16.4 16.5 Amini, K.; Kraatz, H.-B. (2015). "Recent Developments in Biosensor Technologies for Pathogen Detection in Water". JSM Environmental Science & Ecology 3 (1): 9. https://www.jscimedcentral.com/EnvironmentalScience/vol3issue1.php.
- ↑ Dzulkurnain, Nurul Akmaliah; Mokhtar, Marliyana; Rashid, Jahwarhar Izuan Abdul; Knight, Victor Feizal; Wan Yunus, Wan Md Zin; Ong, Keat Khim; Mohd Kasim, Noor Azilah; Mohd Noor, Siti Aminah (15 August 2021). "A Review on Impedimetric and Voltammetric Analysis Based on Polypyrrole Conducting Polymers for Electrochemical Sensing Applications" (in en). Polymers 13 (16): 2728. doi:10.3390/polym13162728. ISSN 2073-4360. PMC PMC8401594. PMID 34451266. https://www.mdpi.com/2073-4360/13/16/2728.
- ↑ Yunus, Sami; Jonas, Alain M.; Lakard, Boris (2013), Roberts, Gordon C. K., ed., "Potentiometric Biosensors" (in en), Encyclopedia of Biophysics (Berlin, Heidelberg: Springer Berlin Heidelberg): 1941–1946, doi:10.1007/978-3-642-16712-6_714, ISBN 978-3-642-16711-9, http://link.springer.com/10.1007/978-3-642-16712-6_714
- ↑ Bahadır, Elif Burcu; Sezgintürk, Mustafa Kemal (2 January 2016). "A review on impedimetric biosensors" (in en). Artificial Cells, Nanomedicine, and Biotechnology 44 (1): 248–262. doi:10.3109/21691401.2014.942456. ISSN 2169-1401. https://www.tandfonline.com/doi/full/10.3109/21691401.2014.942456.
- ↑ Daniels, Jonathan S.; Pourmand, Nader (1 June 2007). "Label-Free Impedance Biosensors: Opportunities and Challenges" (in en). Electroanalysis 19 (12): 1239–1257. doi:10.1002/elan.200603855. PMC PMC2174792. PMID 18176631. https://onlinelibrary.wiley.com/doi/10.1002/elan.200603855.
- ↑ Tajik, Somayeh; Dourandish, Zahra; Jahani, Peyman Mohammadzadeh; Sheikhshoaie, Iran; Beitollahi, Hadi; Shahedi Asl, Mehdi; Jang, Ho Won; Shokouhimehr, Mohammadreza (2021). "Recent developments in voltammetric and amperometric sensors for cysteine detection" (in en). RSC Advances 11 (10): 5411–5425. doi:10.1039/D0RA07614G. ISSN 2046-2069. PMC PMC8694840. PMID 35423079. http://xlink.rsc.org/?DOI=D0RA07614G.
- ↑ 22.0 22.1 Renaud-Young, M.; Mayall, R.M.; Salehi, V. et al. (2019). "Development of an ultra-sensitive electrochemical sensor for Δ9-tetrahydrocannabinol (THC) and its metabolites using carbon paper electrodes". Electrochimica Acta 307: 351–359. doi:10.1016/j.electacta.2019.02.117. ISSN 0013-4686.
- ↑ Sivashanmugan, Kundan; Squire, Kenneth; Tan, Ailing; Zhao, Yong; Kraai, Joseph Abraham; Rorrer, Gregory L.; Wang, Alan X. (25 March 2019). "Trace Detection of Tetrahydrocannabinol in Body Fluid via Surface-Enhanced Raman Scattering and Principal Component Analysis". ACS Sensors 4 (4): 1109–1117. doi:10.1021/acssensors.9b00476. ISSN 2379-3694. http://dx.doi.org/10.1021/acssensors.9b00476.
- ↑ Comeau, Zachary J.; Boileau, Nicholas T.; Lee, Tiah; Melville, Owen A.; Rice, Nicole A.; Troung, Yen; Harris, Cory S.; Lessard, Benoît H. et al. (27 August 2019). "On-the-Spot Detection and Speciation of Cannabinoids Using Organic Thin-Film Transistors". ACS Sensors 4 (10): 2706–2715. doi:10.1021/acssensors.9b01150. ISSN 2379-3694. http://dx.doi.org/10.1021/acssensors.9b01150.
- ↑ Amini, Kaveh; Kraatz, Heinz-Bernhard (1 March 2015). "Recent advances and developments in monitoring biological agents in water samples" (in en). Reviews in Environmental Science and Bio/Technology 14 (1): 23–48. doi:10.1007/s11157-014-9351-5. ISSN 1572-9826. https://doi.org/10.1007/s11157-014-9351-5.
- ↑ Amini, Kaveh; Kraatz, Heinz-Bernhard (14 July 2016). "Toll-like receptors for pathogen detection in water: challenges and benefits". International Journal of Environmental Analytical Chemistry 96 (9): 836–844. doi:10.1080/03067319.2016.1209661. ISSN 0306-7319. https://doi.org/10.1080/03067319.2016.1209661.
- ↑ Jadon, N.; Jain, R.; Sharma, S. et al. (2016). "Recent trends in electrochemical sensors for multianalyte detection – A review". Talanta 161: 894–916. doi:10.1016/j.talanta.2016.08.084. ISSN 0039-9140.
- ↑ 28.0 28.1 28.2 28.3 Lu, Dingqiang; Lu, Fuping; Pang, Guangchang (2016/10). "A Novel Tetrahydrocannabinol Electrochemical Nano Immunosensor Based on Horseradish Peroxidase and Double-Layer Gold Nanoparticles" (in en). Molecules 21 (10): 1377. doi:10.3390/molecules21101377. ISSN 1420-3049. PMC PMC6274132. PMID 27763523. https://www.mdpi.com/1420-3049/21/10/1377.
- ↑ 29.0 29.1 29.2 Zhang, Q.; Berg, D.; Mugo, S.M. (2019). "Molecularly imprinted carbon based electrodes for tetrahydrocannabinol sensing". Inorganic Chemistry Communications 107: 107459. doi:10.1016/j.inoche.2019.107459. ISSN 1387-7003.
- ↑ 30.0 30.1 Canfarotta, Francesco; Czulak, Joanna; Guerreiro, Antonio; Cruz, Alvaro Garcia; Piletsky, Sergey; Bergdahl, Gizem Ertürk; Hedström, Martin; Mattiasson, Bo (1 November 2018). "A novel capacitive sensor based on molecularly imprinted nanoparticles as recognition elements" (in en). Biosensors and Bioelectronics 120: 108–114. doi:10.1016/j.bios.2018.07.070. https://linkinghub.elsevier.com/retrieve/pii/S0956566318305803.
- ↑ 31.0 31.1 Goodwin, Alexander; Banks, Craig E.; Compton, Richard G. (1 June 2006). "Graphite Micropowder Modified with 4-Amino-2,6-diphenylphenol Supported on Basal Plane Pyrolytic Graphite Electrodes: Micro Sensing Platforms for the Indirect Electrochemical Detection of Δ9-Tetrahydrocannabinol in Saliva". Electroanalysis 18 (11): 1063–1067. doi:10.1002/elan.200603518. ISSN 1040-0397. http://dx.doi.org/10.1002/elan.200603518.
- ↑ Suresh, Raghavv Raghavender; Lakshmanakumar, Muthaiyan; Arockia Jayalatha, J. B. B.; Rajan, K. S.; Sethuraman, Swaminathan; Krishnan, Uma Maheswari; Rayappan, John Bosco Balaguru (1 May 2021). "Fabrication of screen-printed electrodes: opportunities and challenges" (in en). Journal of Materials Science 56 (15): 8951–9006. doi:10.1007/s10853-020-05499-1. ISSN 0022-2461. http://link.springer.com/10.1007/s10853-020-05499-1.
- ↑ 33.0 33.1 33.2 Mishra, Rupesh K.; Sempionatto, Juliane R.; Li, Zhanhong; Brown, Christopher; Galdino, Nathalia M.; Shah, Rushabh; Liu, Shuyang; Hubble, Lee J. et al. (1 May 2020). "Simultaneous detection of salivary Δ9-tetrahydrocannabinol and alcohol using a Wearable Electrochemical Ring Sensor" (in en). Talanta 211: 120757. doi:10.1016/j.talanta.2020.120757. https://linkinghub.elsevier.com/retrieve/pii/S0039914020300485.
- ↑ 34.0 34.1 Nissim, Rita; Compton, Richard G (1 December 2015). "Absorptive stripping voltammetry for cannabis detection" (in en). Chemistry Central Journal 9 (1): 41. doi:10.1186/s13065-015-0117-0. ISSN 1752-153X. PMC PMC4493815. PMID 26155306. https://bmcchem.biomedcentral.com/articles/10.1186/s13065-015-0117-0.
- ↑ 35.0 35.1 Wanklyn, Ceri; Burton, Dan; Enston, Emma; Bartlett, Carrie-Ann; Taylor, Sarah; Raniczkowska, Aleksandra; Black, Murdo; Murphy, Lindy (1 December 2016). "Disposable screen printed sensor for the electrochemical detection of delta-9-tetrahydrocannabinol in undiluted saliva" (in en). Chemistry Central Journal 10 (1): 1. doi:10.1186/s13065-016-0148-1. ISSN 1752-153X. PMC PMC4722664. PMID 26807144. https://bmcchem.biomedcentral.com/articles/10.1186/s13065-016-0148-1.
- ↑ 36.0 36.1 Stevenson, Hunter; Bacon, Amanda; Joseph, Kathleen Mary; Gwandaru, Wilma Ruth Wanjiku; Bhide, Ashlesha; Sankhala, Devangsingh; Dhamu, Vikram Narayanan; Prasad, Shalini (3 September 2019). "A Rapid Response Electrochemical Biosensor for Detecting Thc In Saliva" (in en). Scientific Reports 9 (1): 12701. doi:10.1038/s41598-019-49185-y. ISSN 2045-2322. PMC PMC6722119. PMID 31481686. https://www.nature.com/articles/s41598-019-49185-y.
Notes
This presentation is faithful to the original, with only a few minor changes to presentation. Some grammar and punctuation was cleaned up to improve readability. In some cases important information was missing from the references, and that information was added. The discussion about driving under the influence of THC was deemed too abrupt, and a few extra sentences and a citation were added to help the introduction flow better into discussion about electrochemical sensors. Additionally, the original has an introduction section that leads directly into a conclusion section, which is awkward; in this version, content has been divided up into more sections to better segue from the introduction to the conclusion. This version also provides additional relevant citations in the discussion of electrochemical sensor modes.