Journal:Laboratory biosafety guidance related to coronavirus disease (COVID-19)
| Full article title | Laboratory biosafety guidance related to coronavirus disease (COVID-19): Interim guidance, 19 March 2020 |
|---|---|
| Author(s) | World Health Organization; Blacksell, Stuart; Summermatter, Kathrin; Kojima, Kazunobu; Zinsky, Rica; Ogloi, Zsofia |
| Author affiliation(s) | Mahidol Oxford Tropical Medicine Research Unit, University of Bern's Institute for Infectious Diseases, World Health Organization |
| Year published | 2020 |
| Volume and issue | 2020.2 |
| Page(s) | 1–11 |
| Distribution license | Attribution-NonCommercial-ShareAlike 3.0 IGO |
| Website | https://apps.who.int/iris/handle/10665/331500 |
| Download | https://apps.who.int/iris/bitstream/handle/10665/331500/WHO-WPE-GIH-2020.2-eng.pdf (PDF) |
Background
|
|
This LIMSwiki article is out-of-date as of May 2020. The WHO document Laboratory biosafety guidance related to coronavirus disease (COVID-19): Interim guidance, 13 May 2020 supersedes this article's guidance. Please consult the latest WHO guidance for more information. |
The purpose of this document is to provide interim guidance on laboratory biosafety related to the testing of clinical specimens of patients that meet the case definition of the novel pathogen identified in Wuhan, China, that is, coronavirus disease 2019 or COVID-19. The World Health Organization (WHO) will revise these recommendations as necessary.
The following are highlights of COVID-19 laboratory biosafety, discussed in more detail herein:
- All procedures must be performed based on risk assessment and only by personnel with demonstrated capability, in strict observance of any relevant protocols at all times.
- Initial processing (before inactivation) of all specimens should take place in a validated biological safety cabinet (BSC) or primary containment device.
- Non-propagative diagnostic laboratory work (e.g., sequencing, nucleic acid amplification test [NAAT]) should be conducted at a facility using procedures equivalent to biosafety level two (BSL-2).
- Propagative work (e.g., virus culture, isolation or neutralization assays) should be conducted at a containment laboratory with inward directional airflow (BSL-3).
- Appropriate disinfectants with proven activity against enveloped viruses should be used (e.g., hypochlorite [bleach], alcohol, hydrogen peroxide, quaternary ammonium compounds, and phenolic compounds).
- Patient specimens from suspected or confirmed cases should be transported as UN 3373, “Biological Substance, Category B.” Viral cultures or isolates should be transported as Category A, UN 2814, “Infectious Substance, Affecting Humans."
Laboratory biosafety
It is essential to ensure that health laboratories adhere to appropriate biosafety practices. Any testing for the presence of the virus responsible for COVID-19 or of clinical specimens from patients meeting the suspected case definition[1] should be performed in appropriately equipped laboratories, by staff trained in the relevant technical and safety procedures. National guidelines on laboratory biosafety should be followed in all circumstances. For general information on laboratory biosafety guidelines, see the WHO's Laboratory Biosafety Manual, Third Edition[2] (a fourth edition is still in revision[3])
Key points
- Each laboratory should conduct a local (that is, institutional) risk assessment to ensure it is competent to safely perform the intended testing with appropriate risk control measures in place.
- When handling and processing specimens, including blood for serological testing, laboratory practices and procedures that are basic to good microbiological practices and procedures (GMPP) should be followed (see Annex I and the associated video series for more).
- The handling and processing of specimens from cases with suspected or confirmed COVID-19 infection that are intended for additional laboratory tests, such as haematology or blood gas analysis, should follow local guidelines for processing potentially infectious material.
- Non-propagative diagnostic laboratory work, including sequencing and NAAT, on clinical specimens from patients who are suspected or confirmed to be infected with COVID-19, should be conducted adopting the practices and procedures of core requirements[a], (see Annex I), and an appropriate selection of heightened control measures[b], as informed by the local risk assessment. In the interim, the use of BSL-2—as described in the third edition of the WHO's Laboratory Biosafety Manual[2]—remains appropriate until the fourth edition replaces it.
- Handling of material with high concentrations of live virus (such as when performing virus propagation, virus isolation, or neutralization assays) or large volumes of infectious materials should be performed only by properly trained and competent personnel in laboratories capable of meeting additional essential containment requirements and practices, that is, BSL-3.
- Initial processing (before inactivation) of all specimens, including those for sequencing and NAAT, should take place in an appropriately maintained and validated BSC or primary containment device.
- Appropriate disinfectants with proven activity against enveloped viruses should be used for the recommended contact time, at the correct dilution, and within the expiry date after the working solution is prepared.
- All technical procedures should be performed in a way that minimizes the generation of aerosols and droplets.
- Appropriate personal protective equipment (PPE), as determined by a detailed risk assessment, should be worn by all laboratory personnel handling these specimens.
- Patient specimens from suspected or confirmed cases should be transported as UN 3373, “Biological Substance, Category B.” Viral cultures or isolates should be transported as Category A, UN 2814, “Infectious Substance, Affecting Humans."[4]
Recommendations addressing minimal/essential working conditions associated with specific manipulations in laboratory settings
The additional recommendations provided in this section address the minimal/essential working conditions associated with specific manipulations in laboratory settings.
1. Risk assessment
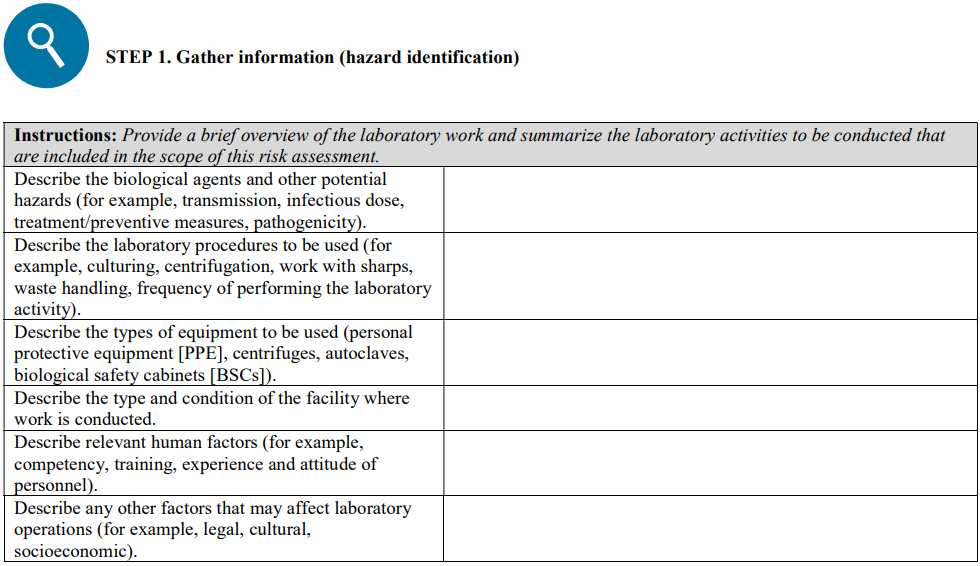
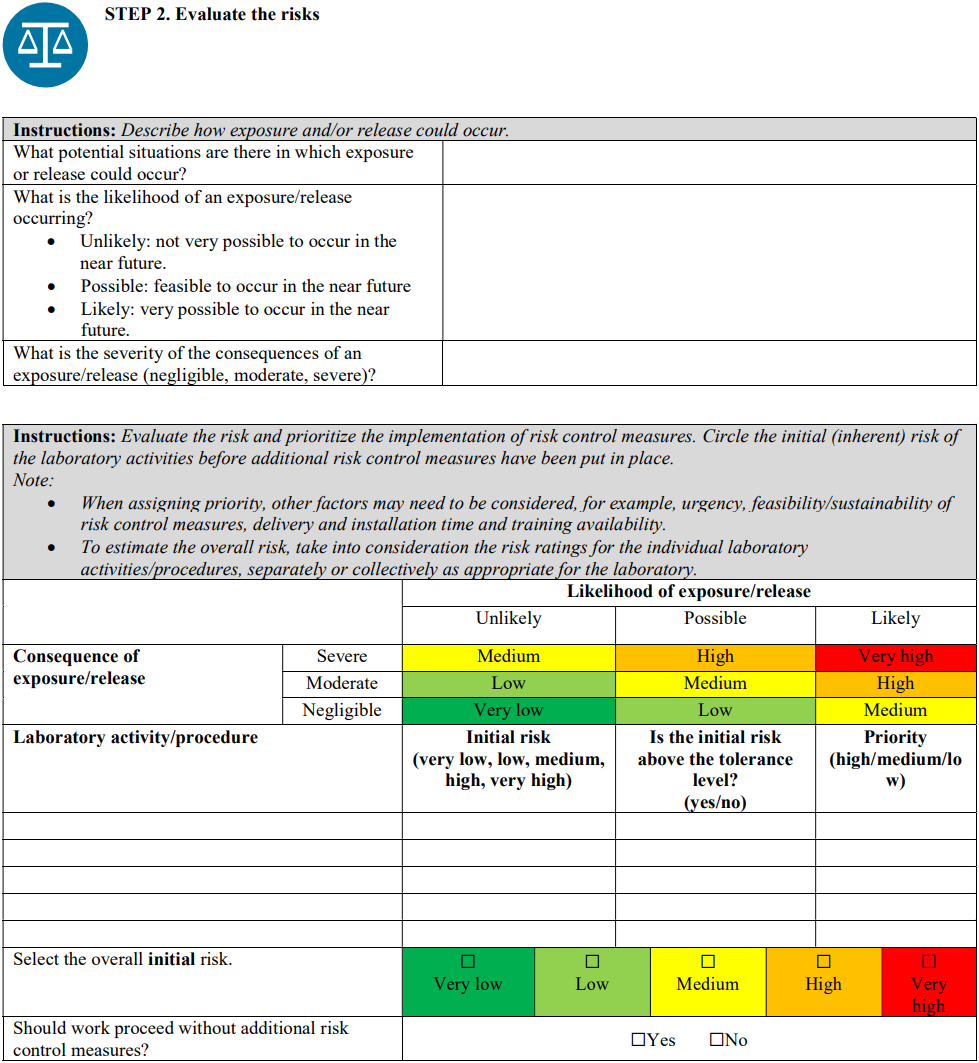
Risk assessment is a systematic process of gathering information and evaluating the likelihood and consequences of exposure to or release of workplace hazard(s) and determining the appropriate risk control measures to reduce the risk to an acceptable level. It is important to note that hazards alone do not pose a risk to humans or animals. Consideration therefore must also be given to the types of equipment used and the procedure(s) that will be performed with the biological agent.
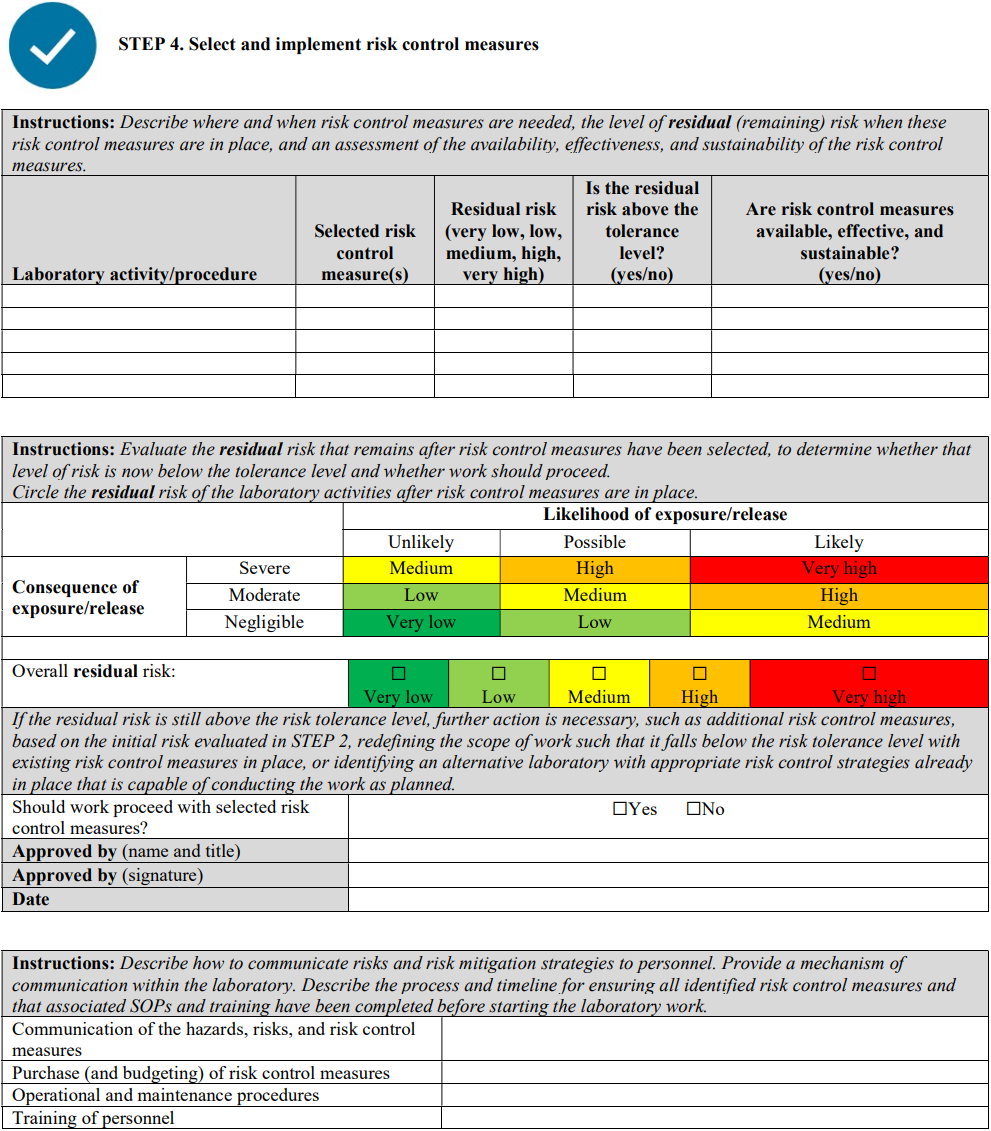
It is highly recommended to start by performing a local risk assessment for each process step, that is, from sample collection, sample reception, clinical testing, and polymerase chain reaction (PCR) to virus isolation (only when and where applicable). Certain hazards will then be considered for each process step, such as aerosol exposure during sample processing; eye splash during sample processing; infectious culture material spill; and leaking sample (in the case of sample reception), with an assessed grade of risk. For each identified risk, appropriate risk control measures—including but not limited to the following recommendations—should be selected and implemented to mitigate the residual risks to an acceptable level.
A risk assessment template is provided in Annex II; this is intended to serve as an example and to facilitate the process.
2. Routine laboratory procedures, including non-propagative diagnostic work and PCR analysis
Non-culture-based diagnostic laboratory work and PCR analysis on clinical specimens from patients who are suspected or confirmed to be infected with the virus responsible for COVID-19 should be conducted adopting practices and procedures described for conventional clinical and microbiology laboratories, as described in the core requirements (see Annex I).
However, all manipulations of potentially infectious materials, including those that may cause splashes, droplets, or aerosols of infectious materials (e.g., loading and unloading of sealed centrifuge cups, grinding, blending, vigorous shaking or mixing, sonic disruption, opening of containers of infectious materials whose internal pressure may be different from the ambient pressure) should be performed in appropriately maintained and validated BSCs or primary containment devices, by personnel with demonstrated capability.
Examples of routine laboratory procedures include:
- diagnostic testing of serum; blood (including haematology and clinical chemistry); respiratory specimens such as nasopharyngeal and oropharyngeal swabs, sputum and/or endotracheal aspiration or bronchoalveolar lavage; stool; or other specimens
- routine examination of mycotic and bacterial cultures developed from respiratory tract specimens (Note: When handling and processing specimens, core requirements [see Annex I], including GMPP, should be followed at all times, including but not limited to those under the following subheadings. More details are explained and demonstrated in the WHO's biosafety video series.[5])
3. Use of appropriate disinfectants
While little is known about this novel virus, the comparable genetic characteristics between the virus responsible for COVID-19 and MERS-CoV (Middle East respiratory syndrome) suggest that the COVID-19 virus may be susceptible to disinfectants with proven activity against enveloped viruses, including sodium hypochlorite (bleach; for example, 1000 parts per million [ppm] (0.1%) for general surface disinfection and 10 000 ppm (1%) for disinfection of blood spills); 62-71% ethanol; 0.5% hydrogen peroxide; quaternary ammonium compounds; and phenolic compounds, if used according to the manufacturer’s recommendations. Other biocidal agents such as 0.05–0.2% benzalkonium chloride or 0.02% chlorhexidine digluconate can be less effective.
Particular attention should be paid not only to the selection of the disinfectant but also the contact time (for example, 10 minutes), dilution (that is, concentration of the active ingredient) and expiry date after the working solution is prepared.
Human coronaviruses in general are known to persist on inanimate surfaces such as metal, glass, or plastic for up to a maximum of nine days.[6]
4. Viral isolation
Unless a country decides otherwise, considering the newly acquired knowledge and effective preventive measures described above, viral isolation on clinical specimens from patients who are suspected or confirmed to be infected with the virus responsible for COVID-19 should be performed only in laboratories capable of meeting the following additional containment criteria:
- has a controlled ventilation system that maintains inward directional airflow into the laboratory room;
- ensures exhaust air from the laboratory room is not recirculated to other areas within the building (Air must be HEPA (high-efficiency particulate air) filtered, if reconditioned and recirculated within the laboratory. When exhaust air from the laboratory is discharged to the outdoors, it must be dispersed away from occupied buildings and air intakes. This air should be discharged through HEPA filters.);
- has a dedicated hand-wash sink;
- has appropriately maintained and validated BSCs for performing all manipulations of infectious or potentially infectious materials;
- ensures laboratory workers wear protective equipment (This includes disposable gloves; solid-front or wrap-around gowns, scrub suits, or coveralls with sleeves that fully cover the forearms; head coverings; shoe covers or dedicated shoes; and eye protection such as goggles or a face shield. Risk assessment should inform the use of respiratory protection such as fit-tested particulate respirators of standardized quality like the E.U.'s FFP2, the U.S.'s NIOSH-certified N95 or equivalent, or higher protection.); and
- performs centrifugation of specimens using sealed centrifuge rotors or sample cups that are loaded and unloaded in a BSC.
5. Additional risks associated with virus isolation studies
Certain experimental procedures may carry additional risks of virus mutations with possible increased pathogenicity and/or transmissibility, or viruses with altered antigenicity or drug susceptibility. Specific risk assessments should be conducted, and specific risk-reduction measures adopted, before any of the following procedures are conducted:
- coinfection of cell cultures with different coronaviruses, or any procedures that may result in a coinfection
- culture of viruses in the presence of antiviral drugs
- deliberate genetic modification of viruses
6. Work with animals infected with the virus responsible for COVID-19
The following activities require an animal facility rated to BSL-3 and associated work practices, as detailed in the third edition of the WHO's Laboratory Biosafety Manual[2]:
- inoculation of animals for potential recovery of the agent from specimens of the virus responsible for COVID-19
- any protocol involving animal inoculation for confirmation and/or characterization of putative agents of the COVID-19 virus
7. Referral of specimens to laboratories with appropriate containment measures in place
Laboratories that are not able to meet the above biosafety recommendations should consider transferring specimens to national, regional, or international referral laboratories with COVID-19-detection capacity that can meet the biosafety requirements.
Packaging and shipment
All materials transported within and between laboratories should be placed in a secondary container to minimize the potential for breakage or a spill. An example includes transfer of materials from the BSC to an incubator and vice versa. Specimens leaving the BSC should have their surface decontaminated. Detailed guidance is provided in the WHO's biosafety video series.[5], in particular "Good Microbiological Practices and Procedures (GMPP) 7: Transport."
Transport of specimens within national borders should comply with national regulations. Cross-boundary transport of specimens of the virus responsible for COVID-19 should follow the United Nations' model regulations Technical Instructions for the Safe Transport of Dangerous Goods by Air (Doc 9284) of the International Civil Aviation Organization[7] for airlifted transport, as well as any other applicable regulations, depending on the mode of transport being used.
More information may be found in the WHO's Guidance on Regulations for the Transport of Infectious Substances 2019–2020[4] A summary on transport of infectious substances can also be found in Tool box 4 of the WHO's handbook Managing Epidemics: Key Facts about Major Deadly Diseases.[8]
Patient specimens from suspected or confirmed cases should be transported as UN 3373, “Biological Substance, Category B” when they are transported for diagnostic or investigational purposes. Viral cultures or isolates should be transported as Category A, UN 2814, “Infectious Substance, Affecting Humans."[4] All specimens being transported (whether UN 3373 or UN 2814) should have appropriate packaging, labeling, and documentation, as described in the documents mentioned earlier.
Annex I: Core requirements
1. Good microbiological practice and procedure (GMPP)
Best practices
The following best practices are to be used:
- Never store food, drink, or personal items such as coats and bags in the laboratory. Activities such as eating, drinking, smoking, and applying cosmetics are only to be performed outside the laboratory.
- Never put materials such as pens, pencils or gum in the mouth while inside the laboratory, regardless of having gloved hands or not.
- Thoroughly wash hands[9], preferably with warm running water and soap, after handling any biological material—including animals—before leaving the laboratory, and any time contamination is known or suspected to be present on the hands.
- Ensure open flames or heat sources are never placed near flammable supplies and are never left unattended.
- Ensure that coverings are placed over any cuts or broken skin prior to entering the laboratory.
- Ensure that, prior to entry into the laboratory, supplies of laboratory equipment and consumables—including reagents, PPE, and disinfectants—are sufficient and appropriate for the activities being performed. * Ensure supplies are stored appropriately (that is, according to storage instructions) and safely to reduce the chance of accidents and incidents such as spills, trips, or falls for laboratory personnel.
- Ensure proper labeling of all biological agents and chemical and radioactive material.
- Protect written documents from contamination using barriers (such as plastic coverings), particularly those that may need to be removed from the laboratory.
- Ensure work is performed with care, in a timely manner, and without rushing. Working when fatigued should be avoided.
- Keep the work area tidy, clean, and free of clutter and materials that are not necessary for the work being done.
- Prohibit the use of earphones, which can distract personnel and prevent equipment or facility alarms from being heard.
- Appropriately cover or remove any jewelry that could tear glove material, easily become contaminated or act as a fomite for infection. If worn regularly, cleaning and decontamination of jewelry or spectacles should be considered.
- Refrain from using mobile electronic devices (e.g., mobile telephones, tablets, laptops, flash drives, memory sticks, cameras, or other portable devices, including those used for DNA/RNA sequencing) when not specifically required for the laboratory procedures being performed.
- Keep mobile electronic devices in areas where they could not easily become contaminated or act as a fomite for infection. Where close proximity of such devices to biological agents is unavoidable, ensure they are either protected by a physical barrier or decontaminated before leaving the laboratory.
Technical procedures
The following technical procedures are to be used:
- Avoid inhalation of biological agents. Use good techniques to minimize the formation of aerosols and droplets when manipulating specimens.
- Avoid ingestion of biological agents and contact with the skin and eyes.
- Wear disposable gloves at all times when handling specimens.
- Avoid contact of gloved hands with the face.
- Shield or otherwise protect the mouth, eyes, and face during procedures where splashes may occur.
- Wherever possible, replace any glassware with plasticware.
- For work requiring scissors, use scissors with blunt or rounded ends in preference to those with pointed ends.
- Handle all sharps, syringes, and needles, if necessary, with care so as to prevent injury and injection of biological agents.
- Use ampoule openers for safe handling of ampoules.
- Never re-cap, clip, or remove needles from disposable syringes.
- Dispose of any sharps materials (e.g., needles, needles combined with syringes, blades, broken glass) in puncture-proof or puncture-resistant containers fitted with sealed covers.
- Prevent the dispersal of biological agents by doing the following: 1. Discard specimens and cultures for disposal in leak-proof containers with the tops appropriately secured before disposal in dedicated waste containers. 2. Consider opening tubes with a disinfectant-soaked pad/gauze. 3. Decontaminate work surfaces with a suitable disinfectant at the end of work procedures and when any material is spilled or obviously contaminated. 4. Ensure the disinfectant is efficacious against the pathogen being handled and is left in contact with infectious waste materials for sufficient time to effect complete inactivation.
2. Personnel competence and training
General familiarization and awareness training
General training should include an introduction to laboratory layout, codes of practice, local guidelines, safety manuals, risk assessments, legislative requirements, and emergency response procedures.
Job-specific training
Training requirements may vary depending on the job functions. However, in general, all personnel involved in the handling of biological agents must be trained on GMPP. All relevant competency and proficiency assessments must be used and verified before working independently, followed by regular review and refresher training. When new procedures are to be put in place, training documentation must be updated and the changes communicated to applicable personnel.
Safety and security training
All personnel must be aware of hazards present in the laboratory and their associated risks. Additionally, personnel should be aware of and follow safe working procedures, security measures, and emergency preparedness and response plans.
3. Facility design
The following condiserations should be made with any authorized testing facility:
- Ample space and a designated hand-washing basin must be provided, with appropriate restriction of access.
- Doors must be appropriately labeled, and laboratory walls, floors, and furniture must be smooth, easy to clean, impermeable to liquids, and resistant to the chemicals and disinfectants normally used in the laboratory.
- Laboratory ventilation, where provided (including heating/cooling systems and especially fans/local cooling split-system air-conditioning units [specifically when retrofitted]) should ensure airflows do not compromise safe working. Consideration must be made for resultant airflow speeds and directions, and turbulent airflows should be avoided; this applies also to natural ventilation.
- Laboratory space and facilities must be adequate and appropriate for safe handling and storage of infectious and other hazardous materials, such as chemicals and solvents.
- Facilities for eating and drinking must be provided outside the laboratory, and first-aid-facilities must be accessible.
- Appropriate methods for decontamination of waste, for example disinfectants and autoclaves, must be available close to the laboratory.
- The management of waste must be considered in the laboratory design. Safety systems must cover fire, electrical emergencies, and emergency/incident response facilities, based on risk assessment.
- There must be a reliable and adequate electricity supply and lighting to permit safe exit.
- Emergency situations must be considered in the design, as indicated in the local risk assessment, and should include the geographical/meteorological context.
4. Specimen receipt and storage
Specimens received and stored at the laboratory require the following considerations:
- A specimen received by the laboratory must be accompanied by sufficient information to identify what it is, when and where it was taken or prepared, and which tests and/or procedures (if any) are to be performed.
- Consider unpacking the items in the BSC. Personnel unpacking and receiving specimens must be adequately trained in awareness of the hazards involved, how to adopt necessary precautions according to GMPP described earlier, how to handle broken or leaking containers, and how to handle spills and use disinfectants to manage any contamination.
- Specimens must be stored in containers that are of adequate strength, integrity, and volume to contain the specimen; leakproof when the cap or stopper is correctly applied; made of plastic whenever possible; free of any biological material on the outside of the packaging; correctly labeled, marked, and recorded to facilitate identification; and made of an appropriate material for the type of storage required.
- Inactivation methods must be appropriately validated whenever an inactivation step is used, before transferring the specimens to other areas for further manipulation, such as PCR analysis.
5. Decontamination and waste management
The following decontamination and waste management steps should taken:
- Any surface or material known to be, or potentially be, contaminated by biological agents during laboratory operations must be correctly disinfected to control infectious risks.
- Proper processes for the identification and segregation of contaminated materials must be adopted before decontamination or disposal.
- Where decontamination cannot be performed in the laboratory area or onsite, the contaminated waste must be packaged in an approved (that is, leakproof) manner, for transfer to another facility with decontamination capacity.
6.Personal protective equipment (PPE)
These important considerations should be made in regard to PPE:
- Laboratory coats must be used in laboratories to prevent personal clothing from getting splashed or contaminated by biological agents. Laboratory coats must have long sleeves, preferably with elasticated or fitted cuffs, and must be worn closed. Sleeves should never be rolled up. Coats must be long enough to cover the knees, but not trail on the floor. They should be fastened when worn in the laboratory. Where possible, the fabric of the laboratory coat should be splash-resistant and overlap to provide a solid front. Laboratory coats must only be worn in designated areas. When not in use, they should be stored appropriately; they should not be hung on top of other laboratory coats, or in lockers or on hooks with personal items.
- Appropriate disposable gloves must be worn for all procedures that may involve planned or inadvertent contact with blood, body fluids, or other potentially infectious materials. They must not be disinfected or reused, as exposure to disinfectants and prolonged wear will reduce the integrity of the glove and decrease protection to the user. Gloves should always be inspected before use, to check they are intact.
- Safety glasses, safety goggles, face shields (visors), or other protective devices must be worn whenever it is necessary to protect the eyes and face from splashes, impacting objects, or artificial ultraviolet radiation. Eye protection can be reused but must be regularly cleaned after every use. If splashed, it must be decontaminated with an appropriate disinfectant.
- Footwear must be worn in the laboratory and must be of a design that minimizes slips and trips and can reduce the likelihood of injury from falling objects and exposure to biological agents.
- Respiratory protection is generally not a part of the core requirements. In this particular context, however, a local risk assessment should be conducted to determine whether the use of respiratory protection is needed, especially when procedures that may create aerosols and droplets will be performed outside the BSC. Examples of such procedures include centrifugation or handling leaking samples and procedures in such a way that can cause splashes (e.g., loading and unloading of sealed centrifuge cups, grinding, blending, vigorous shaking or mixing, sonic disruption, and opening of infectious material containers with an internal pressure that may be different from the ambient pressure).
7. Laboratory equipment
When used effectively together with GMPP, the safe use of laboratory equipment will help to minimize the likelihood of exposure of personnel when handling or manipulating biological agents. For that equipment to effectively reduce such risks, laboratory management must ensure sufficient space is provided for its use. An appropriate budget must be available for the equipment’s operation and maintenance, including equipment incorporated into the facility design, which should be accompanied by specifications that outline its safety features. All personnel operating or maintaining a piece of equipment must be properly trained and be able to demonstrate proficiency.
8. Emergency/incident response plan
The following describes the salient points of planning for and reacting to emergencies and incidents in the lab:
- Even when carrying out low-risk work and following all core requirements for biosafety, incidents can still occur. To reduce the likelihood of exposure to or the release of a biological agent, or to reduce the consequences of such incidents, a contingency plan must be developed that provides specific standard operating procedures (SOPs) to be followed in possible emergency scenarios that apply to the work and local environment. Personnel must be trained on these procedures and have periodic refresher training to maintain competency.
- First-aid kits, including medical supplies such as bottled eye washes and bandages, must be available and easily accessible to personnel. These must be checked routinely to make sure products are within their use-by dates and are in sufficient supply.
- All incidents must be reported to the appropriate personnel in a timely manner. A written record of accidents and incidents must be maintained, in line with national regulations where applicable. Any incident must be reported and investigated in a timely manner and used for updating laboratory procedures and emergency response plans.
- Spill kits, including disinfectant, must be easily accessible to personnel. Depending on the size, location, concentration, or volume of the spill, different protocols may be necessary. Written procedures for cleaning and decontaminating spills must be developed for the laboratory and followed by suitably trained personnel.
9. Occupational health
The employing authority, through the laboratory director, must take responsibility for ensuring the health of laboratory personnel is adequately checked and reported. A medical examination or the documentation of health status information of laboratory personnel may be required to ensure it is safe for them to work in the laboratory.
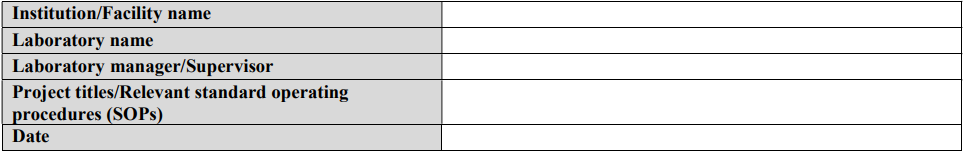
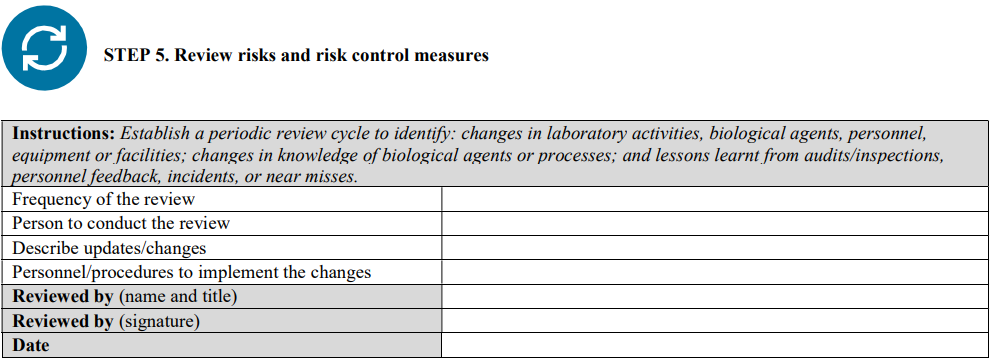
Annex II: Risk assessment template
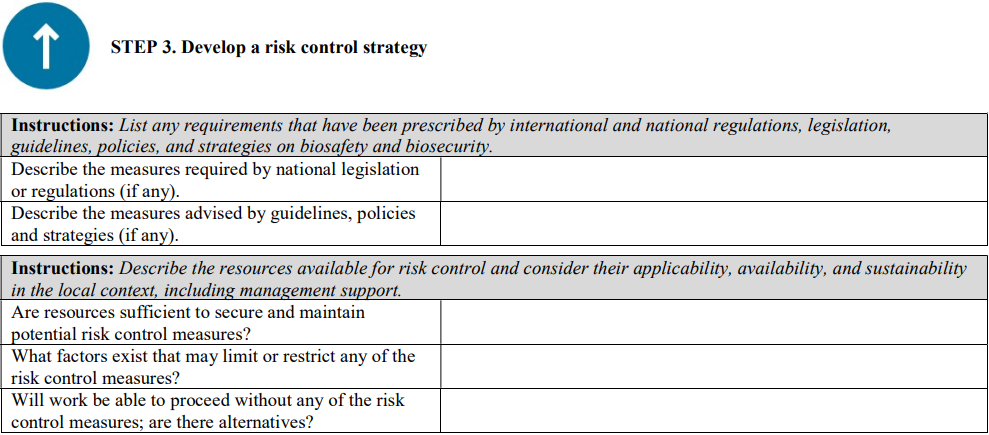
Although a qualitative approach to combining likelihood and severity parameters in a risk matrix is provided as a method for risk evaluation here, it is important to note that quantitative methods—from simple numerical scoring schemes to complex mathematical models—and hybrid (semi-quantitative) methods can also be used for risk evaluation. Laboratories should use a risk-evaluation/assessment method that best meets their unique needs, without excluding the possibility of developing customized evaluation approaches, scoring methods, and definitions of the parameters.
This template was primarily developed for biosafety risk assessment; however, it can also be used for general safety risk assessment of laboratory activities—especially when the biosafety and general safety risks are interlinked (e.g., sample collection and transport)—where appropriate and applicable.
INSTRUCTIONS: When using this template, complete the header information (names, project/SOP titles, dates, etc.) and all sections following the instructions in the grey boxes. The instructions and bullet points in the grey boxes can be copied into the text boxes beneath the instructions and used as prompts to gather and record the necessary site-specific information. The grey instruction boxes can then be deleted, and the text remaining will form a risk assessment draft. This draft must be carefully reviewed, edited as necessary, and approved by the members of the risk assessment team.
Footnotes
- ↑ Core requirements: A set of minimum requirements defined in the upcoming fourth edition of the WHO's Laboratory Biosafety Manual to describe a combination of risk control measures that are both the foundation for, and an integral part of, laboratory biosafety. These measures reflect international standards and best practice in biosafety that are necessary to work safely with biological agents, even where the associated risks are minimal.
- ↑ Heightened control measures: A set of risk control measures that may need to be applied in a laboratory facility because the outcome of a risk assessment indicates that the biological agents being handled and/or the activities to be performed with them are associated with a relatively high risk that cannot be acceptable solely with the core requirements.
Acknowledgements
The following people contributed to the current revision of this guidance: Stuart Blacksell, Mahidol Oxford Tropical Medicine Research Unit, Thailand; Kathrin Summermatter, Institute for Infectious Diseases, University of Bern, Switzerland. From the WHO Health Emergency Programme: Kazunobu Kojima, Rica Zinsky, and Zsofia Igloi.
WHO continues to monitor the situation closely for any changes that may affect this interim guidance. Should any factors change, WHO will issue a further update. Otherwise, this interim guidance document will expire two years after the date of publication.
References
- ↑ World Health Organization (20 March 2020). "Global surveillance for COVID-19 caused by human infection with COVID-19 virus: Interim guidance, 20 March 2020". WHO/2019-nCoV/SurveillanceGuidance/2020.6. World Health Organization. https://apps.who.int/iris/handle/10665/331506.
- ↑ 2.0 2.1 2.2 World Health Organization (2004) (3rd ed.). World Health Organization. ISBN 9241546506.
- ↑ Kojima, K. (7 February 2019). "WHO Laboratory Biosafety Manial Revision Update". SlideShare. World Health Organization. https://www.slideshare.net/MicrobiologySection/who-laboratory-biosafety-manual-revision-update.
- ↑ 4.0 4.1 4.2 World Health Organization (January 2019). "Guidance on regulations for the transport of infectious substances 2019–2020". WHO/WHE/CPI/2019.20. World Health Organization. https://www.who.int/ihr/publications/WHO-WHE-CPI-2019.20/en/.
- ↑ 5.0 5.1 World Health Organization (2005). "Biosafety video series". Strengthening health security by implementing the International Health Regulations (2005). World Health Organization. https://www.who.int/ihr/publications/biosafety-video-series/en/.
- ↑ Kampf, G.; Todt, D.; Pfaender, S.; Steinmann, E. (2020). "Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents". Journal of Hospital Infection 104 (3): 246–51. doi:10.1016/j.jhin.2020.01.022. PMID 32035997.
- ↑ International Civil Aviation Organization (June 2017). "Technical Instructions For The Safe Transport of Dangerous Goods by Air (Doc 9284)". United Nations. https://www.icao.int/safety/DangerousGoods/Pages/technical-instructions.aspx.
- ↑ World Health Organization (2018). Managing Epidemics: Key Facts about Major Deadly Diseases. World Health Organization. pp. 257. ISBN 9789241565530.
- ↑ World Health Organization (October 2006). "How to handrub? How to handwash?" (PDF). World Health Organization. https://www.who.int/gpsc/tools/GPSC-HandRub-Wash.pdf.
Notes
This presentation is faithful to the original, with some changes to presentation, grammar, and punctuation. In some cases important information was missing from the references, and that information was added. Added an external link to WHO's GMPP video series for this version. A citation concerning the fourth edition of WHO's Laboratory Biosafety Manual was added for this version. Per the WHO's licensing agreement, this reproduction acknowledges the World Health Organization as the source, licensed under the CC BY-NC-SA 3.0 IGO license (see the infobox at top for full details).