Journal:FluxCTTX: A LIMS-based tool for management and analysis of cytotoxicity assays data
| Full article title | FluxCTTX: A LIMS-based tool for management and analysis of cytotoxicity assays data |
|---|---|
| Journal | BMC Bioinformatics |
| Author(s) |
Faria-Campos, Alessandra C.; Balottin, Luciene B.; Zuin, Gianlucca; Garcia, Vinicius; Batista, Paulo H.S.; Granjeiro, José M.; Campos, Sérgio V.A. |
| Author affiliation(s) | Universidade Federal de Minas Gerais, INMETRO |
| Primary contact | Email: alessa at dcc dot ufmg dot br |
| Year published | 2015 |
| Volume and issue | 16(Suppl 19) |
| Page(s) | 58 |
| DOI | 10.1186/1471-2105-16-S19-S8 |
| ISSN | 1471-2105 |
| Distribution license | Creative Commons Attribution 4.0 International |
| Website | https://bmcbioinformatics.biomedcentral.com/articles/10.1186/1471-2105-16-S19-S8 |
| Download | https://bmcbioinformatics.biomedcentral.com/track/pdf/10.1186/1471-2105-16-S19-S8 (PDF) |
Abstract
Background: Cytotoxicity assays have been used by researchers to screen for cytotoxicity in compound libraries. Researchers can either look for cytotoxic compounds or screen "hits" from initial high-throughput drug screens for unwanted cytotoxic effects before investing in their development as a pharmaceutical. These assays may be used as an alternative to animal experimentation and are becoming increasingly important in modern laboratories. However, the execution of these assays in large-scale and different laboratories requires, among other things, the management of protocols, reagents, and cell lines used, as well as the data produced, which can be a challenge. The management of all this information is greatly improved by the utilization of computational tools to save time and guarantee quality. However, a tool that performs this task designed specifically for cytotoxicity assays is not yet available.
Results: In this work, we have used a workflow based LIMS — the Flux system — and the Together Workflow Editor as a framework to develop FluxCTTX, a tool for management of data from cytotoxicity assays performed at different laboratories. The main work is the development of a workflow, which represents all stages of the assay and has been developed and uploaded in Flux. This workflow models the activities of cytotoxicity assays performed as described in the OECD 129 Guidance Document.
Conclusions: FluxCTTX presents a solution for the management of the data produced by cytotoxicity assays performed at interlaboratory comparisons. Its adoption will contribute to guarantee the quality of activities in the process of cytotoxicity tests and enforce the use of good laboratory practices (GLP). Furthermore, the workflow developed is complete and can be adapted to other contexts and different tests for management of other types of data.
Keywords: laboratory information management systems, workflow, cytotoxicity tests, OECD129, good laboratory practices, interlaboratory comparison
Background
Cytotoxicity is the quality of being toxic to cells. Examples of toxic agents are some types of venom (e.g., from the puff adder or brown recluse spider). Treating cells with the cytotoxic compound can result in a variety of cell fates: The cells may undergo necrosis, stop actively growing and dividing or activate a genetic program of controlled cell death (apoptosis). Cytotoxicity assays are the tests used by researchers to screen for cytotoxicity in compound libraries. Researchers can either look for cytotoxic compounds, if they are interested in developing a therapeutic that targets rapidly dividing cells, or they can screen "hits" from initial high-throughput drug screens for unwanted cytotoxic effects before investing in their development as a pharmaceutical.
Cytotoxicity tests may be used as a substitute to in vivo tests that use animals. The concept of using in vitro cytotoxicity data to determine the starting doses for rodent acute oral toxicity tests was discussed and evaluated at the International Workshop on In Vitro Methods for Assessing Acute Systemic Toxicity convened in 2000.[1] The approach involves using an IC50 value from an in vitro basal cytotoxicity test with the Registry of Cytotoxicity (RC) regression to predict an LD50 value for use as a starting dose for the Acute Toxic Class (ATC) method or the Up-and-Down Procedure (UDP) test method.[2] Simulations showed that using in vitro cytotoxicity assays to estimate an LD50 to use as a starting dose in the UDP could potentially reduce animal use by 25 to 40 percent. Additionally, several tests have currently demonstrated the efficiency and effectiveness of alternative methods testing to reduce, refine, and/or replace the use of animals in testing.[1][2][3][4][5]
However, to rely on these assays it is very important that quality is assured for all tests through a well-designed quality management system (QMS) which includes tools to register and verify the information regarding the tests. For research laboratories two important QMSs are the management systems from the International Organization for Standardization (ISO) and good laboratory practices (GLP). Good laboratory practices are defined by the Organization for Economic Cooperation and Development (OECD) as "a quality system concerned with the organizational process and the conditions under which non-clinical health and environmental safety studies are planned, performed, monitored, recorded, archived and reported."[6][7] The purpose of the GLP principles is to promote the development of quality test data and provide a tool to ensure a sound approach to the management of laboratory studies, including conduct, reporting and archiving. The GLP principles may be considered as a set of standards for ensuring the quality, reliability and integrity of studies, the reporting of verifiable conclusions and the traceability of data. The ISO management systems provide a model to follow when setting up and operating a laboratory according to a set of internationally recognized standards. They are the result of international expert consensus and therefore offer the benefit of global management experience and good practice.[8]
The recognition of compliance to quality management principles by laboratories can be complex. This process can be slow and costly, potentially requiring changes in laboratory routine. Recognition and maintenance of compliance to these principles is also difficult because it requires that the laboratory maintains a continuing routine of activities that include training of staff, maintenance of facilities and equipment, monitoring document and lab record quality, inspection of calendars and periodic reporting. Manual capture, calculation and verification of raw data result in a tremendous drain on human resources while also jeopardizing the integrity of the information. The administration of paper records is particularly inefficient and expensive, and data cannot be easily integrated with other technologies. As a result, complying with the strict principles of quality systems can be a very time consuming and expensive process. Thus, the use of computer systems capable of addressing the complexity of the regulations, ensuring compliance with current best practice and satisfying the concerns and expectations of the regulators is pressing. The main tool that can assist in this process is the laboratory information management system (LIMS), which allows the recording of lab activities in a complete and easy way. All data are recorded in databases and the relationship between the experimental steps is logged, allowing the traceability of samples and the documentation of chain of custody, reagents and results.[9][10] An extensive list of LIMS can be found nowadays, and several LIMS are currently available as academic, proprietary and open-source solutions. Some examples of these include SQL LIMS[11], LabSoft[12], LabWare[13], FreeLIMS[14], Biotracker[15], Watson[16] and the systems developed by Hendricks[17], Quo[18], Tharayil[19] and Sanchez-Villeda.[20]
One of the main difficulties to implement a LIMS is the fact that each laboratory has a different routine of experimentation that changes over time. Therefore some LIMS developers propose a customization to the LIMS general structure to better meet the needs of the laboratory.[15][16] However, this customization can be time consuming and may sometimes not address all the needs of a specific laboratory, such as those performing the cytotoxicity tests. Moreover, although a significant number of LIMS are available today, none of them addresses specifically the information on cytotoxicity assays and therefore more general ones are used to store these data, but typically the analysis is performed manually through independent analysis tools.[21] Other works mention cytotoxicity assays being managed by in-house LIMS, being therefore unavailable for comparison.[22] There are LIMS that provide some functionality for cytotoxicity data, such as the one provided by Thermo Scientific, which offers an image analysis application.[23] In these cases, however, the LIMS is not used for a complete cytotoxicity analysis, only for part of it.
This work proposes the use of a workflow based LIMS — the Flux system — and the Together Workflow Editor as a framework to develop FluxCTTX, a tool for management of data from cytotoxicity assays performed at different laboratories. The main work is the development of a workflow, which represents all stages of the assay and has been developed and uploaded in Flux. This workflow models the activities of cytotoxicity assays performed as described in the OECD 129 Guidance Document[6], and guarantees that experiments performed adhere to the GLP principles.
Methods
The design and construction of FluxCTTX involved the use of a LIMS (with an associated database server) as an engine and a workflow editor to construct the forms for the interface.
The Flux system
Flux is a LIMS that has been constructed using Java technology and uses MySQL as a database server and Apache Tomcat as a web server. The system has a web interface which is platform-independent and can be accessed using the main browsers (Internet Explorer, Google Chrome, Mozilla Firefox and Safari). Workflow files are uploaded in the system through its web interface. The activities created in the workflow construction are then interpreted by the system as links.
The workflow CTTX has been constructed using the Together Workflow Editor (TWE)[24] in the XML Process Definition Language (XPDL) file format. The Flux system is able to represent different workflows modeled in TWE in the XPDL format according to the needs of each experimental design. In Flux the protocols are defined as processes that are composed of steps, referred to as activities. An activity represents events of a process and, as such, has transitions, actors and rules. This information is represented as attributes in the workflow definition. Therefore, the user can define the characteristics of the attributes of each activity, such as its types, the range of values that each attribute can assume, its formats or even define auto-calculated attributes derived from other attributes. In TWE, inputs and outputs of each activity are also defined, including the relation of these with the experiments. During workflow definition it is also possible to assign to each activity a documentation that contains standard protocols, instruments calibration, procedures and records associated with the activities.
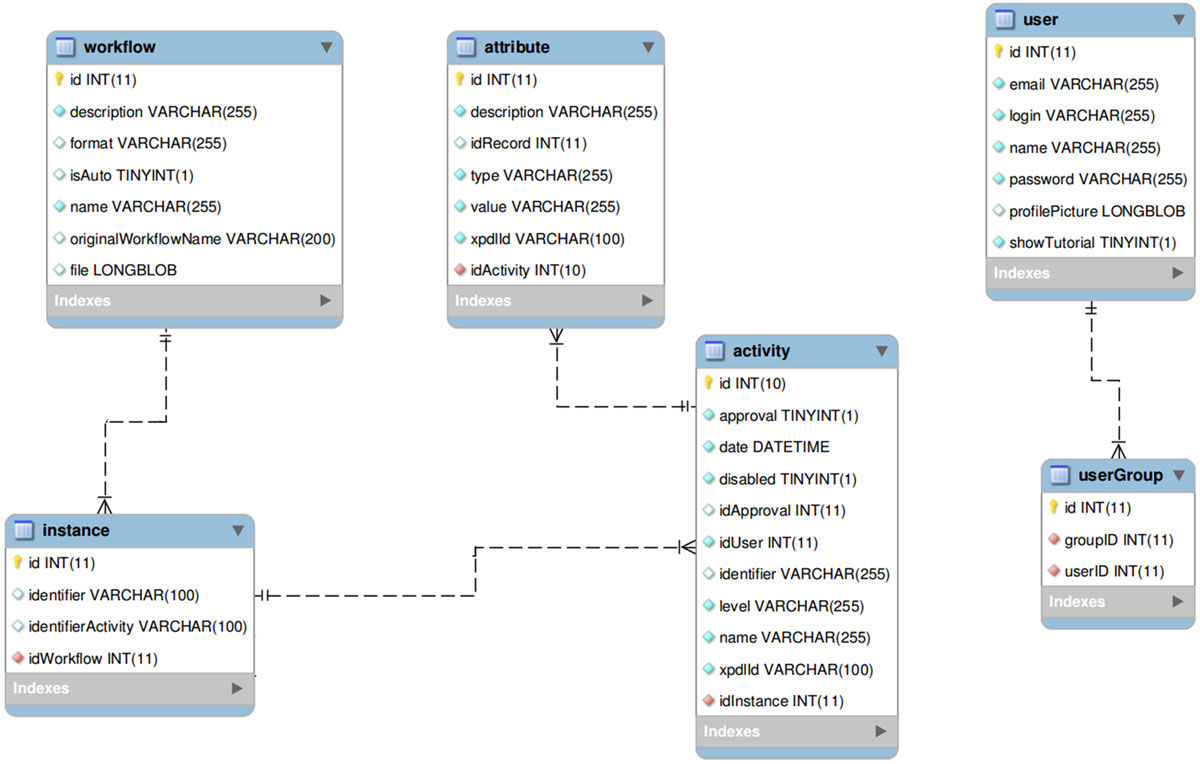
All the information regarding the activities and attributes is stored in a entity-relationship database through an activities table that stores all the data, using metadata to indicate, whenever necessary, to which experiment or activity each data belongs to. A simplified entity-relationship diagram for this is showed in Figure 1.
|
The cytotoxicity assay modeled in FluxCTTX
The cytotoxicity assay modeled in FlucCTTX was the Neutral Red Uptake (NRU) in vitro basal cytotoxicity assay procedure described by the OECD 129 Guidance Document on Using Cytotoxicity Tests for Estimate Starting doses for Acute Oral Systemic Toxicity Tests.[6] The test is based on the ability of viable cells to incorporate and bind neutral red (NR), a supravital dye. Toxicants can alter cell surface or lysosomal membranes that cause cell death and or cell growth inhibition, which decreases the amount of NR retained by the culture. The concentration of NR dye desorbed from the cultured cells is directly proportional to the number of living cells, so cytotoxicity is expressed as a concentration-dependent reduction of the uptake of NR after chemical exposure. The NRU assay uses a 96-well plate format that is reproduced in the system. The light absorption (optical density - OD) at 540 nm within 60 minutes of adding NR is measured using a microtiter plate reader (spectrophotometer) using blank as reference. Values of OD are registered in the system and the averages for OD of the blank are calculated. Two controls are present in the test: (i) a positive control (PC) where the test is performed using sodium dodecyl sulphate (SDS) as a test substance to obtain a complete dose-response curve and (ii) a vehicle control (VC), that consists of a routine culture medium when the test substances are dissolved in culture medium. Cell viability is calculated automatically by the system for each test well as a percent of the mean VC at OD540. The IC50 of the test substance is automatically calculated by the system using the statistical package R, using a Hill function analysis of the replicate cell viability data for each concentration.
The construction of the CTTX workflow
The development of FluxCTTX consisted of the construction of the CTTX workflow and its upload to the LIMS Flux. The instructions for the cytotoxicity assay of the OECD 129 guidance document have been used for the workflow construction. Information regarding the set up of the assay as well as its results has been described in the systems as attributes that are detailed according to the information stored. There are several different types of attributes, each fulfilling a specific need in storing and managing the data. In this workflow each activity corresponds to an experimental step, and its attributes identify the types of information that are generated in this experimental step. The sequence of activities then contain all the steps of assays performed. The attributes in each activity store the specific information of that activity. As a consequence, the workflow has the complete description of the data being managed, all the steps, and, for each step, all the required data. Because of this, the FluxCTTX system has been fully developed by specifying the workflow, without need to change the code of the LIMS. Other workflows, or adaptations to this workflow can be developed by changing the workflow files in the editor, without changing the Flux system. The information managed by the FluxCTTX system is completely contained in its workflow; the Flux system is the engine that drives the workflow. It is used to exhibit the information contained in the workflow, that is self-contained in the same way as a spreadsheet or text file. The file contains the data and all of the formulas that are required to understand it, and FluxCTTX is the engine to understand and process these data. Moreover, for those attributes that represent results of calculations, the system performs those, automatically preventing human errors.
Results
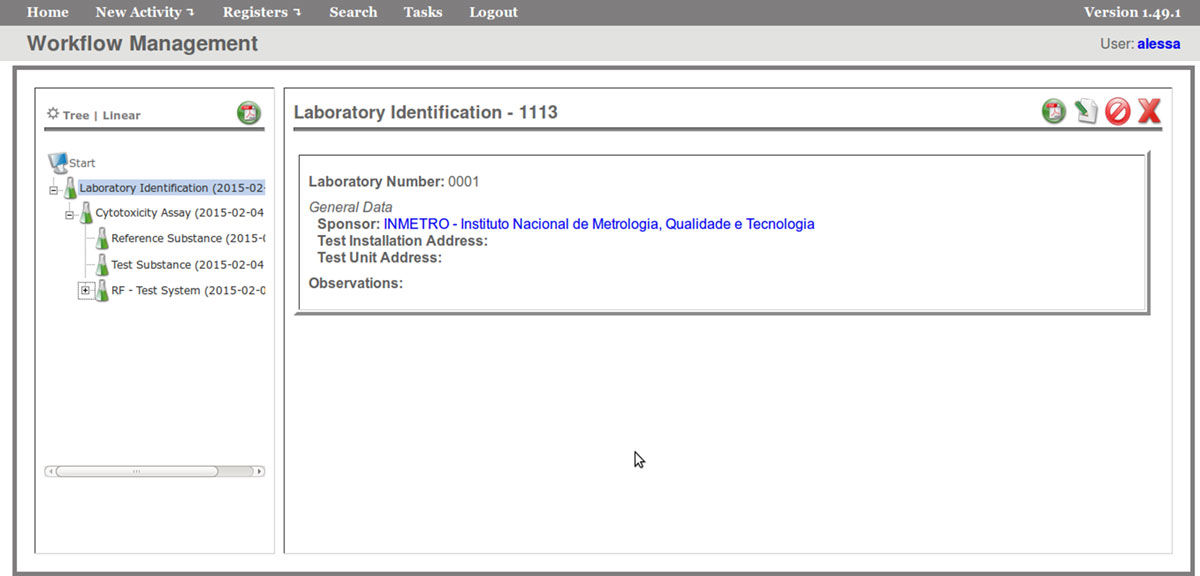
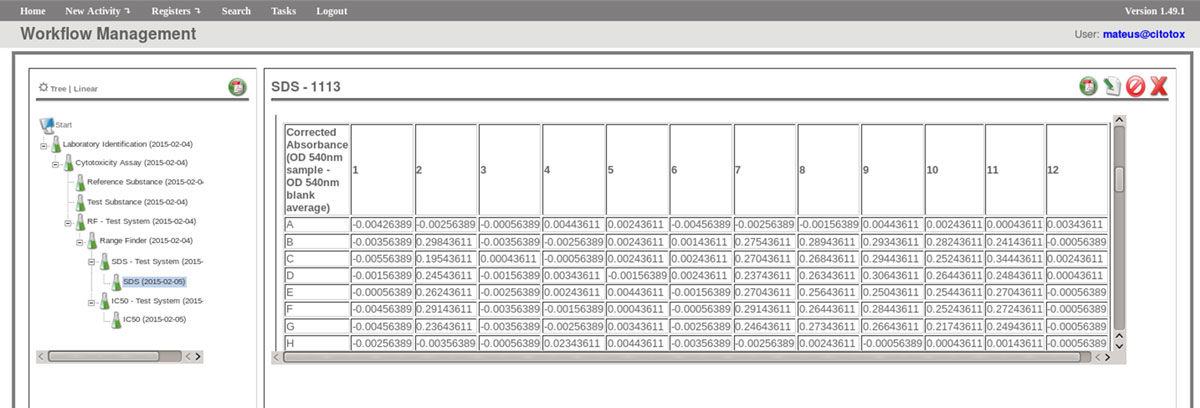
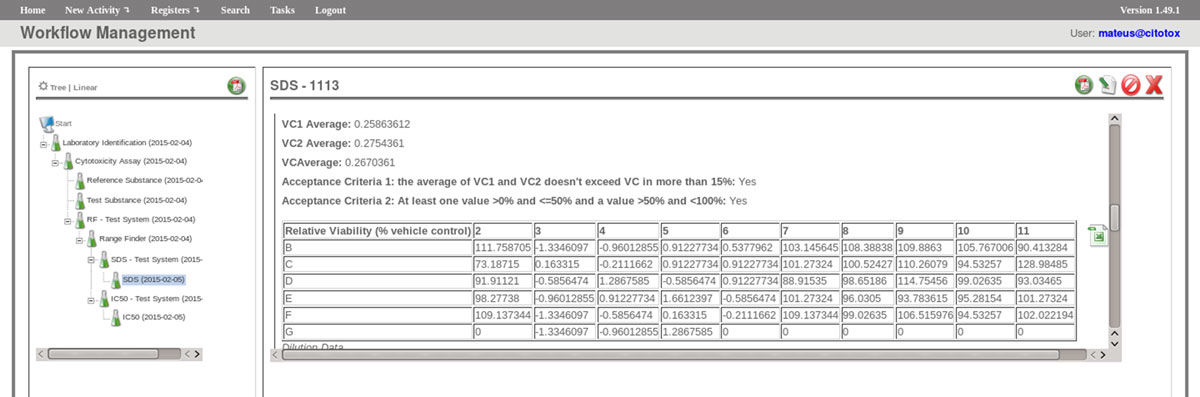
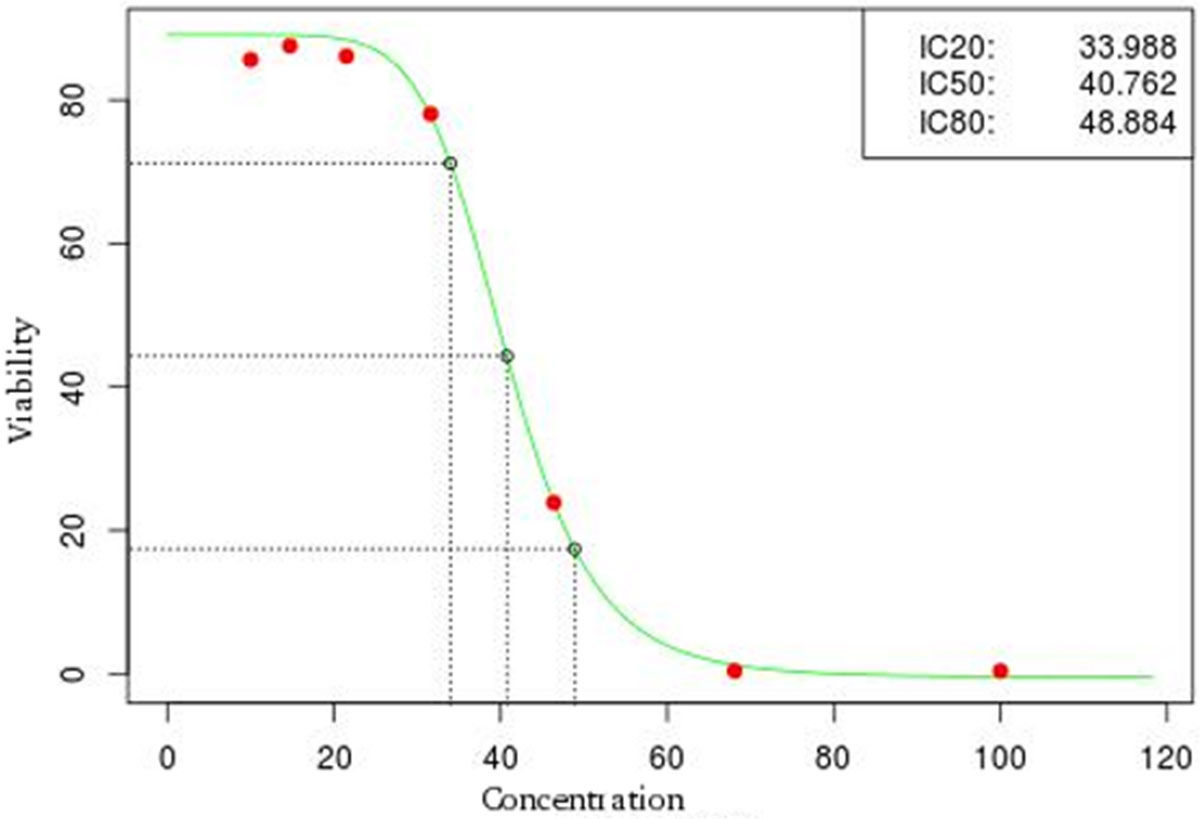
The FluxCTTX workflow has been developed using TWE and has four main activities, representing the steps of the OECD129 protocol, and three child activities (Figure 2). Laboratory identification, the first activity, identifies the laboratory, the test performed, reagents used and team involved in the procedure. The following activities keep the information regarding the range finder for the test substance, the test itself (IC50) and the positive control. These forms have the information on samples used and the values of optical density (OD) at 540 nm for the tests, including the corrected OD, calculated by the system subtracting the OD values obtained for the blank (average) from those obtained for the test substance (Figure 3). The workflow also shows the values for cell viability calculated automatically by the system (Figure 4). Moreover, the values of IC50 are also calculated automatically by the system using the R package, and the graphical representation of the calculations is exhibited (Figure 5).
|
|
|
|
The first test of FluxCTTX has been made by inviting five different laboratories in Brazilian institutions that perform cytotoxicity assays to participate in an interlaboratory comparison where the information regarding the assays was uploaded in the FluxCTTX system. The users in these laboratories inserted the data of the experiments performed and checked the calculations performed by the system with those performed manually using Microsoft Excel. The results matched. A total of 24 assays were performed and the data from those uploaded in the FluxCTTX system. The results are summarized in Table 1.
| ||||||||||||||||||||||||||||||||||||||||
The director of the study had access to all the data inserted in the system and was able to compare the data inserted by the five different laboratories in a very efficient and precise way.
Discussion
FluxCTTX has been used in an interlaboratory comparison by five different laboratories located in three different Brazilian states. Each laboratory has different coordinators and uses different experimentation techniques. Because of this diversity, in previous laboratory comparisons each had performed the experiments slightly different than the others, and comparing the results had been difficult. In addition to that, some laboratories had not previously complied with the GLP principles. Due to the lack of standardization in laboratory procedures it became difficult not only to compare results but also to identify the reasons why results varied among them.
IC50 values have been computed showing that FluxCTTX can accurately compute automatically the required values directly from the experimental data. Previously, in these labs the experimental data had to be manually input in a separate statistical analysis tool, potentially leading to errors. Moreover, different tools calculated the IC50 slightly differently, leading to results that could not be directly compared. It is important to notice that because there are no other cytotoxicity LIMS available to the laboratories, a direct comparison between FluxCTTX and another cytotoxicity LIMS is not possible. In some cases the LIMS is not available to external users.[22] In other cases, the systems available only automate part of the analysis[21][23], and a manual component equivalent to the manual analysis mentioned above must be performed.
All results of the comparison have been inserted in the system, except for one. One laboratory did not follow the OECD 129 standard protocol for the experiments and reported their results without using the FluxCTTX system. The IC50 values reported by this group have had the largest variance of all experiments, indicating that adherence to the standard protocol increases the accuracy of the experiments. This is not a definite indication, since the number of experiments was too small, but it is a clear one, since the only lab that did not follow the protocol was the only one that was unable to analyze their results in the system, and the one with the less accurate results. Notice that without an integrated method of managing the data and reporting the results, the coordinator of the comparison would have to rely only on reports sent by the laboratories. In most cases the reports would have been different, and a comparison would be difficult. In particular, it would be very difficult to identify that a lab did not follow the standard protocol, and the relation between adherence to protocol and more accurate results might not have been found.
Of the experiments uploaded in the system, not all have been considered satisfactory. FluxCTTX managed, however, to store data from all experiments and helped to identify the better ones by computing the IC50 values. It is important not only to identify the best experiments, but also to store the unsatisfactory ones for comparison purposes. We are currently sifting through the data stored to identify the reasons for the success or failure of experiments. If common patterns exist that indicate success or failure of experiments, this information can be used to suggest actions in future experiments to increase the likelihood of a successful experiment.
Conclusion
In this work we present the development of the FluxCTTX system, a LIMS designed to manage and analyze cytotoxicity assays performed in accordance to GLP principles and the protocols of the OECD 129 guidance document. FluxCTTX manages experimental data and analyzes them, computing IC50 values and suggesting if the experiments are satisfactory or not. FluxCTTX has been successfully used in an interlaboratory comparison with five different laboratories, making it simpler to follow the standard protocol, to report results, and to compare these results. Future comparisons including a larger number of laboratories are planned, which should provide more data to be analyzed not only to assess the effectiveness of FluxCTTX but also to help identify the best experimental procedures.
The FluxCTTX system will be available at http://syrah.luar.dcc.ufmg.br/fluxcttx.
List of abbreviations
CTTX: cytotoxicity
GLP: good laboratory practice
LIMS: laboratory information management system
OECD: Organization for Economic Cooperation and Development
SDS: sodium dodecyl sulphate
TWE: Together Workflow Editor
XPDL: XML Process Definition Language
Declarations
Acknowledgements
The authors wish to thank INMETRO and the Brazilian agencies CAPES (Coordena¸cão de Aperfei¸coamento de Pessoal de Nível Superior), FAPEMIG (Funda¸cão de Amparo à Pesquisa do Estado de Minas Gerais), FAPERJ (Funda¸cão de Amparo à Pesquisa do Estado do Rio de janeiro) and CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico) for financial support and Mateus Lopes Teixeira for helping with the figures.
Declarations
Publication charges for this article have been funded by INMETRO and Computer Science Department at Federal University of Minas Gerais.
This article has been published as part of BMC Bioinformatics Volume 16 Supplement 19, 2015: Brazilian Symposium on Bioinformatics 2014. The full contents of the supplement are available online at http://www.biomedcentral.com/bmcbioinformatics/supplements/16/S19
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
ACFC, GZ and VG constructed and tested the CTTX workflow XPDL files. PHB, VG and SVAC developed and implemented the LIMS Flux. LBB and JMG inserted the data on FluxCTTX, coordinated and performed the interlaboratory comparison. All authors have helped in preparing the manuscript.
References
- ↑ 1.0 1.1 Interagency Coordinating Committee on the Validation of Alternative Methods (August 2001). "Report of the International Workshop on In Vitro Methods for Assessing Acute Systemic Toxicity" (PDF). National Institute of Environmental Health Sciences. https://ntp.niehs.nih.gov/iccvam/docs/acutetox_docs/finalrpt/finalall0801.pdf.
- ↑ 2.0 2.1 Spielmann, H.; Genschow, E.; Liebsch, M.; Halle, W. (1999). "Determination of the Starting Dose for Acute Oral Toxicity (LD50) Testing in the Up and Down Procedure (UDP) From Cytotoxicity Data". Alternatives to Laboratory Animals 27 (6): 957-66. PMID 25490464.
- ↑ Stokes, W.S.; Casati, S.; Strickland, J.; Paris, M. (2008). "Neutral red uptake cytotoxicity tests for estimating starting doses for acute oral toxicity tests". Current Protocols in Toxicology 36 (20.4): 20.4.1–20.4.20. doi:10.1002/0471140856.tx2004s36. PMID 20967741.
- ↑ EURL ECVAM (December 2014). "EURL ECVAM strategy to replace, reduce and refine the use of animals in the assessment of acute mammalian systemic toxicity". European Commission. pp. 46. https://eurl-ecvam.jrc.ec.europa.eu/eurl-ecvam-strategy-papers/strategy-acute-mammalian-systemic-toxicity.
- ↑ National Institute of Environmental Health Sciences. "Alternatives to Animal Testing". National Institutes of Health. https://www.niehs.nih.gov/health/topics/science/sya-iccvam/.
- ↑ 6.0 6.1 6.2 Organisation for Economic Co-operation and Development (20 July 2010). "Guidance Document on Using Cytotoxicity Tests to Estimate Starting Doses for Acute Oral Systemic Toxicity Tests" (PDF). pp. 54. http://www.oecd.org/officialdocuments/publicdisplaydocumentpdf/?cote=env/jm/mono(2010)20&doclanguage=en.
- ↑ dos Santos, P.E. (2010). "Boas Práticas de Laboratório (BPL): Uma questão de qualidade" (PDF). Revista Intertox de Toxicologia, Risco Ambiental e Sociedade 3 (2). http://www.revistarevinter.com.br/minhas-revistas/2010/v-3-n-2-2010-volume-3-numero-2-junho-de-2010-sao-paulo/80-boas-praticas-de-laboratorio-bpl-uma-questao-de-qualidade/file.
- ↑ "Management system standards". International Organization for Standardization. https://www.iso.org/management-system-standards.html.
- ↑ Hinton, M.D. (1994). Laboratory Information Management Systems: Development and Implementation for a Quality Assurance Laboratory. Marcel Dekker, Inc. ISBN 0824794583.
- ↑ Paszko, C.; Turner, E. (2001). Laboratory Information Management Systems. CRC Press. pp. 242. ISBN 9780824705213.
- ↑ "LabLite". LabLite, LLC. http://www.lablite.com/.
- ↑ "LabSoft LIMS". Computing Solutions, Inc. http://www.labsoftlims.com/.
- ↑ "LabWare". LabWare, Inc. http://www.labware.com/en.
- ↑ "FreeLIMS". SourceForge. Slashdot Media. https://sourceforge.net/projects/freelims/.
- ↑ 15.0 15.1 "Biotracker LIMS". Ocimum Biosolutions Ltd. https://www.ocimumbio.com/biotrackertm/.
- ↑ 16.0 16.1 "Watson LIMS Software". Thermo Fisher Scientific. https://www.thermofisher.com/order/catalog/product/INF-21000.
- ↑ Hendricks, R.W.; Learn, M.R. (2003). "A laboratory information management system (LIMS) for an academic microchip fabrication facility". Proceedings of the 15th Biennial University/Government/Industry Microelectronics Symposium, 2003. doi:10.1109/UGIM.2003.1225703.
- ↑ Quo, C.F.; Wu, B.; Wang, M.D. (2005). "Development of a laboratory information system for cancer collaboration projects". Conference Proceedings from the Annual International Conference of the IEEE Engineering in Medicine and Biology Society 3: 2859-62. doi:10.1109/IEMBS.2005.1617070. PMID 17282839.
- ↑ Tharayil, S.M.; Kalbfleisch, T.; Kumar, A. (2007). "Service-Oriented Laboratory Information Management System for Life Sciences Research". IEEE International Conference on Services Computing, 2007. doi:10.1109/SCC.2007.103.
- ↑ Sanchez-Villeda, H.; Schroeder, S.; Polacco, M. et al. (2003). "Development of an integrated laboratory information management system for the maize mapping project". Bioinformatics 19 (16): 2022-30. PMID 14594706.
- ↑ 21.0 21.1 Carballo, J.L.; Hernández-Inda, Z.L.; Pérez, P. et al. (2002). "A comparison between two brine shrimp assays to detect in vitro cytotoxicity in marine natural products". BMC Biotechnology 2: 17. PMC PMC130034. PMID 12270067. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC130034.
- ↑ 22.0 22.1 "Safety/Toxicology". BIOalternatives. Archived from the original on 21 January 2015. https://web.archive.org/web/20150121061259/http://www.bioalternatives.com/customized-services/safety-toxicology/.
- ↑ 23.0 23.1 "Thermo Scientific Cellomics Cell Health Profiling V4 BioApplication Guide" (PDF). Thermo Fisher Scientific. http://www.med.cam.ac.uk/wp-content/uploads/2016/02/CellHealthProfiling_V4_LC06070800.pdf.
- ↑ "Together WfMC XPDL and OMG BPMN Java Workflow Editor". Together Teamsolutions Co., Ltd. http://www.together.at/prod/workflow/twe.
Notes
This presentation is faithful to the original, with only a few minor changes to presentation. In some cases important information was missing from the references, and that information was added. Some grammar were corrected when necessary. Several of the original URLs have changed since 2015 and have been updated to current or replaced with an archived version.