Difference between revisions of "User:Shawndouglas/sandbox/sublevel1"
Shawndouglas (talk | contribs) |
Shawndouglas (talk | contribs) |
||
| Line 1: | Line 1: | ||
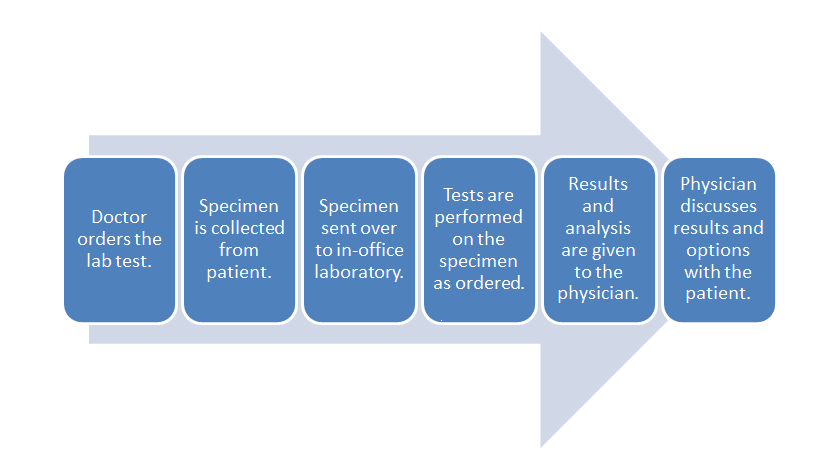
[[File: | [[File:POLWorkflow.png|620px|thumb|'''Figure 5.''' This diagram depicts a generic workflow of diagnostic testing for a patient, from the doctor ordering the test, the laboratory running its tests (with its own workflow), and the doctor finally receiving the results and passing them on to the patient.]]It took three chapters, but now—with all that background in-hand—we can finally address how [[cloud computing]] relates to the various type of [[Laboratory|laboratories]] spread across multiple sectors. Let's first begin with what an average software-enabled laboratory's data [[workflow]] might look. We can then address the benefits of cloud computing within the scope of that workflow and the regulations that affect it. Afterwards, we can examine different deployment approaches and the associated costs and benefits. | ||
In | Let's turn to a broad workflow using diagnostic testing of a patient as an example. Figure 5 depicts a generic workflow for this process, with a doctor first ordering a laboratory test for a patient. This may be done through a [[Computerized physician order entry]] (CPOE) module found in an [[electronic health record]] (EHR) system, or through some other electronic system. In turn, a test order appears in the electronic system of the laboratory. This might occur in the lab's own CPOE module, usually integrated in some way with a [[laboratory information system]] (LIS) or a [[laboratory information management system]] (LIMS). The patient then provides the necessary specimens, either at a remote location, with the specimens then getting shipped to the main lab, or directly at the lab where the testing it to be performed. Whenever the specimens are received at the laboratory, they must be checked-in or registered in the LIS or LIMS, as part of an overall responsibility of maintaining [[chain of custody]]. At this delivery point, the laboratory's own processes and workflows take over, with the LIS or LIMS aiding in the assurance that the specimens are accurately tracked from delivery to final results getting reported (and then some, if specimen retention times are necessary). | ||
That internal laboratory workflow will look slightly different for each clinical lab, but a broad set of steps will be realized with each lab. One or more specimens get registered in the system, barcoded labels get generated, tests and any other preparative activities are scheduled for the specimens, laboratory personnel are assigned, the scheduled workloads are completed, the results get analyzed and verified, and if nothing is out of specification (OOS) or abnormal, the results get logged and placed in a deliverable report. Barring any mandated specimen retention times, the remaining specimen material is properly disposed. At this point, most of the clinical laboratory's obligations are met; the resulting report is sent to the ordering physician, who in turn shares the results with the original patient. | |||
When we look at these workflows today, a computerized component is usually associated with them. From the CPOE and EHR to the LIS and LIMS, software systems and other [[laboratory automation]] have been an increasing area of focus for improving laboratory efficiencies, reducing human error, shortening turnaround times, and improving regulatory compliance.<ref name="WeinsteinAutomation07">{{cite journal |title=Automation in the Clinical Pathology Laboratory |journal=North Carolina Medical Journal |author=Weinstein, M.; Smith, G. |volume68 |issue=2 |pages=130–31 |year=2007 |doi=10.18043/ncm.68.2.130}}</ref><ref name="PrasadTrends12">{{cite journal |title=Trends in laboratory information management system |journal=Chemometrics and Intelligent Laboratory Systems |author=Prasad, P.J.; Bodhe, G.L. |volume=118 |pages=187–92 |year=2012 |doi=10.1016/j.chemolab.2012.07.001}}</ref><ref name="CaseyDigital20">{{cite journal |title=Digital transformation risk management in forensic science laboratories |journal=Forensic Science International |author=Casey, E.; Souvignet, T.R. |volume=316 |at=110486 |year=2020 |doi=10.1016/j.forsciint.2020.110486}}</ref> In the laboratory, the LIS and LIMS are able to electronically maintain an [[audit trail]] of sample movement, analyst activities, record alteration, and much more. The modern LIS and LIMS are also able to limit access to data and functions based on assigned role, track versioning of documents, manage scheduling, issue alarms and alerts for OOS results, maintain training records, and much more. | |||
Yet with all the benefits of software use in the laboratory comes additional risks involving the security and protection of the data that software manages, transmits, and receives. Under some circumstances, these risks may become more acute by moving software systems to cloud computing environments. We broadly discussed many of those risks in the previous chapter. Data security and regulatory risk demands that labs moving into the cloud carefully consider the appropriate and necessary use of user access controls, networking across multitenancy or shared infrastructures, and encryption and security controls offered by the cloud service provider (CSP). Technology risk demands that labs embracing cloud have staff with a constant eye on what's changing in the industry and what tools can extend the effective life span of their cloud implementations. Operational risk demands that labs integrating cloud solutions into their workflows have redundancy plans in place for how to mitigate risks from physical disasters and other threats to their data hosted in public and private clouds. Vendor risk demands that labs thoroughly vet their cloud service providers, those providers' long-term stability, and their fall-back plans should they suffer a major business loss. And finally, financial risk demands that labs turning to the cloud should have staff who are well-versed in financial management and can effectively understand the current and future costs of implementing cloud solutions in the lab. | |||
However, | However, this talk of risk shouldn't scare away laboratory staff considering a move to the cloud; the cloud can benefit automated laboratories in many ways. However, it does require a thoughtful, organization-wide approach to managing the risks (as discussed in Chapter 3). Let's take a look at the benefits a laboratory could realize from moving to cloud-based solutions. | ||
==References== | ==References== | ||
{{Reflist|colwidth=30em}} | {{Reflist|colwidth=30em}} | ||
Revision as of 23:22, 3 February 2022
It took three chapters, but now—with all that background in-hand—we can finally address how cloud computing relates to the various type of laboratories spread across multiple sectors. Let's first begin with what an average software-enabled laboratory's data workflow might look. We can then address the benefits of cloud computing within the scope of that workflow and the regulations that affect it. Afterwards, we can examine different deployment approaches and the associated costs and benefits.
Let's turn to a broad workflow using diagnostic testing of a patient as an example. Figure 5 depicts a generic workflow for this process, with a doctor first ordering a laboratory test for a patient. This may be done through a Computerized physician order entry (CPOE) module found in an electronic health record (EHR) system, or through some other electronic system. In turn, a test order appears in the electronic system of the laboratory. This might occur in the lab's own CPOE module, usually integrated in some way with a laboratory information system (LIS) or a laboratory information management system (LIMS). The patient then provides the necessary specimens, either at a remote location, with the specimens then getting shipped to the main lab, or directly at the lab where the testing it to be performed. Whenever the specimens are received at the laboratory, they must be checked-in or registered in the LIS or LIMS, as part of an overall responsibility of maintaining chain of custody. At this delivery point, the laboratory's own processes and workflows take over, with the LIS or LIMS aiding in the assurance that the specimens are accurately tracked from delivery to final results getting reported (and then some, if specimen retention times are necessary).
That internal laboratory workflow will look slightly different for each clinical lab, but a broad set of steps will be realized with each lab. One or more specimens get registered in the system, barcoded labels get generated, tests and any other preparative activities are scheduled for the specimens, laboratory personnel are assigned, the scheduled workloads are completed, the results get analyzed and verified, and if nothing is out of specification (OOS) or abnormal, the results get logged and placed in a deliverable report. Barring any mandated specimen retention times, the remaining specimen material is properly disposed. At this point, most of the clinical laboratory's obligations are met; the resulting report is sent to the ordering physician, who in turn shares the results with the original patient.
When we look at these workflows today, a computerized component is usually associated with them. From the CPOE and EHR to the LIS and LIMS, software systems and other laboratory automation have been an increasing area of focus for improving laboratory efficiencies, reducing human error, shortening turnaround times, and improving regulatory compliance.[1][2][3] In the laboratory, the LIS and LIMS are able to electronically maintain an audit trail of sample movement, analyst activities, record alteration, and much more. The modern LIS and LIMS are also able to limit access to data and functions based on assigned role, track versioning of documents, manage scheduling, issue alarms and alerts for OOS results, maintain training records, and much more.
Yet with all the benefits of software use in the laboratory comes additional risks involving the security and protection of the data that software manages, transmits, and receives. Under some circumstances, these risks may become more acute by moving software systems to cloud computing environments. We broadly discussed many of those risks in the previous chapter. Data security and regulatory risk demands that labs moving into the cloud carefully consider the appropriate and necessary use of user access controls, networking across multitenancy or shared infrastructures, and encryption and security controls offered by the cloud service provider (CSP). Technology risk demands that labs embracing cloud have staff with a constant eye on what's changing in the industry and what tools can extend the effective life span of their cloud implementations. Operational risk demands that labs integrating cloud solutions into their workflows have redundancy plans in place for how to mitigate risks from physical disasters and other threats to their data hosted in public and private clouds. Vendor risk demands that labs thoroughly vet their cloud service providers, those providers' long-term stability, and their fall-back plans should they suffer a major business loss. And finally, financial risk demands that labs turning to the cloud should have staff who are well-versed in financial management and can effectively understand the current and future costs of implementing cloud solutions in the lab.
However, this talk of risk shouldn't scare away laboratory staff considering a move to the cloud; the cloud can benefit automated laboratories in many ways. However, it does require a thoughtful, organization-wide approach to managing the risks (as discussed in Chapter 3). Let's take a look at the benefits a laboratory could realize from moving to cloud-based solutions.
References
- ↑ Weinstein, M.; Smith, G. (2007). "Automation in the Clinical Pathology Laboratory". North Carolina Medical Journal (2): 130–31. doi:10.18043/ncm.68.2.130.
- ↑ Prasad, P.J.; Bodhe, G.L. (2012). "Trends in laboratory information management system". Chemometrics and Intelligent Laboratory Systems 118: 187–92. doi:10.1016/j.chemolab.2012.07.001.
- ↑ Casey, E.; Souvignet, T.R. (2020). "Digital transformation risk management in forensic science laboratories". Forensic Science International 316: 110486. doi:10.1016/j.forsciint.2020.110486.