Difference between revisions of "User:Shawndouglas/sandbox/sublevel1"
Shawndouglas (talk | contribs) |
Shawndouglas (talk | contribs) |
||
| Line 1: | Line 1: | ||
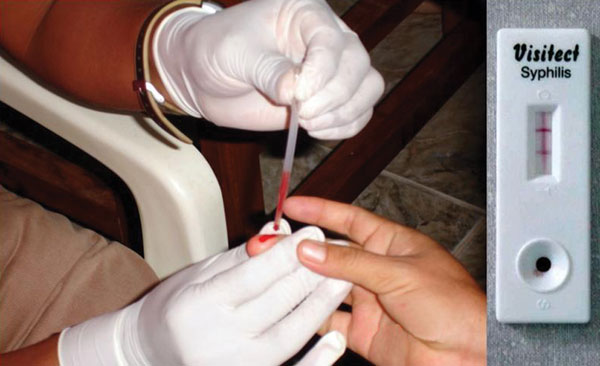
[[File:Rapid point-of-care syphilis test-CDC.jpg|right|thumb|Point-of-care devices such as this rapid syphilis test are commonly used in the POL.]]The physician office lab, or POL, is a physician-, partnership-, or group-maintained [[laboratory]] that performs medical diagnostic tests or examines specimens in order to diagnose, prevent, and/or treat a disease or impairment in a patient as part of the physician practice.<ref name="CMSPOLDef">{{cite web |url=https://www.cms.gov/Regulations-and-Guidance/Guidance/Manuals/downloads/clm104c16.pdf |format=PDF |title=Chapter 16 - Laboratory Services |work=Medicare Claims Processing Manual |publisher=Centers for Medicare and Medicaid Services |date=09 March 2021 |accessdate=18 November 2021}}</ref><ref name="WasPOLEP">{{cite web |url=https://www.wadsworth.org/regulatory/polep |title=Physician Office Laboratory Evaluation Program (POLEP) |publisher=Wadsworth Center New York State Department of Health |accessdate=18 November 2021}}</ref> The POL shows up in primary care physician offices as well as the offices of specialists like urologists, hematologists, gynecologists, and endocrinologists. In many countries like the United States, the POL is considered a clinical laboratory and is thus regulated by federal, state, and/or local laws affecting such laboratories.<ref name="WasPOLEP" /><ref name="CDPHLabs">{{cite web |url=http://www.cdph.ca.gov/programs/lfs/Documents/POL-FAQ.pdf |archiveurl=https://web.archive.org/web/20161229143212/http://www.cdph.ca.gov/programs/lfs/Documents/POL-FAQ.pdf |format=PDF |title=Physician Office Laboratories or Clinics - Frequently Asked Questions about Clinical Laboratory Licensing and Registration |publisher=California Department of Public Health |date=May 2008 |archivedate=29 December 2016 |accessdate=03 January 2020}}</ref> In October 2021, the [[Centers for Medicare and Medicaid Services]] (CMS) reported 41% of all CLIA-approved laboratories in the United States (130,335) were physician office laboratories.<ref name="CMSDec13Count">{{cite web |url=https://www.cms.gov/Regulations-and-Guidance/Legislation/CLIA/downloads/factype.pdf |format=PDF |title=Laboratories by Type of Facility |publisher=Centers for Medicare and Medicaid Services |date=October 2021 |accessdate=18 November 2021}}</ref> However, as of 2014, POLs were estimated to be processing only about nine percent of all clinical laboratory tests.<ref name="KalHow14Arch">{{cite web |url=http://www.kaloramainformation.com/article/2014-11/How-and-Where-IVD-Will-Find-Growth-Global-POL-Market-%E2%80%93-Part-2 |archiveurl=https://web.archive.org/web/20150417204832/http://www.kaloramainformation.com/article/2014-11/How-and-Where-IVD-Will-Find-Growth-Global-POL-Market-%E2%80%93-Part-2 |title=How and Where IVD Will Find Growth in the Global POL Market – Part 2 |publisher=Kalorama Information |date=November 2014 |archivedate=17 April 2015 |accessdate=03 January 2020}}</ref> | |||
[[File: | |||
Testing and reporting at a POL, at least in the U.S., is largely concentrated on the realm of waived CLIA testing. As of October 2021, 68% of the POLs in the United States were primarily running CLIA waived tests.<ref name="CMS13Enroll">{{cite web |url=https://www.cms.gov/Regulations-and-Guidance/Legislation/CLIA/Downloads/statupda.pdf |format=PDF |title=Enrollment, CLIA exempt states, and certification of accreditation by organization |author=Centers for Medicare and Medicaid Services, Division of Laboratory Services |date=October 2021 |accessdate=18 November 2021}}</ref> CLIA test complexity has three levels: high, moderate, and waived.<ref name="CDCTestCom">{{cite web |url=https://www.cdc.gov/clia/test-complexities.html |title=Clinical Laboratory Improvement Amendments (CLIA): Test complexities |author=Centers for Disease Control and Prevention |date=06 August 2018 |accessdate=18 November 2021}}</ref> Waived tests are simple to perform and have a relatively low risk of an incorrect test result. Moderately complex tests include tests like provider performed microscopy (PPM), which requires the use of a microscope during the office visit. Providers that want to perform PPM tests must be qualified to do so under CLIA regulations.<ref name="CDCTestCom" /> High-complexity tests require the most regulation. These tests are the most complicated and run the highest risk of an inaccurate result, as determined during the [[Food and Drug Administration]] (FDA) pre-market approval process. Tests may come from the manufacturer with their complexity level on them, or one can search the FDA database to determine the complexity of the test.<ref name="CDCTestCom" /> | |||
Commonly performed tests include<ref name="UHOxInOffice">{{cite web |url=https://www.oxhp.com/secure/policy/in_office_laboratory_testing_and_procedures_list.pdf |format=PDF |title=UnitedHealthcare Oxford's in-office laboratory testing and procedures list |author=UnitedHealthcare Oxford |date=01 January 2018 |accessdate=18 November 2021}}</ref>: | |||
* urine analysis | |||
* urine pregnancy | |||
* blood occult | |||
* | * glucose blood | ||
* | * pathology consultation during surgery | ||
* | * crystal identification by microscope | ||
* | * sperm identification and analyses | ||
* bilirubin total | |||
* blood gasses | |||
* complete blood count | |||
* bone marrow smear | |||
* blood bank services | |||
* transfusion medicine | |||
==References== | ==References== | ||
{{Reflist|colwidth=30em}} | {{Reflist|colwidth=30em}} | ||
Revision as of 23:07, 21 January 2022
The physician office lab, or POL, is a physician-, partnership-, or group-maintained laboratory that performs medical diagnostic tests or examines specimens in order to diagnose, prevent, and/or treat a disease or impairment in a patient as part of the physician practice.[1][2] The POL shows up in primary care physician offices as well as the offices of specialists like urologists, hematologists, gynecologists, and endocrinologists. In many countries like the United States, the POL is considered a clinical laboratory and is thus regulated by federal, state, and/or local laws affecting such laboratories.[2][3] In October 2021, the Centers for Medicare and Medicaid Services (CMS) reported 41% of all CLIA-approved laboratories in the United States (130,335) were physician office laboratories.[4] However, as of 2014, POLs were estimated to be processing only about nine percent of all clinical laboratory tests.[5]
Testing and reporting at a POL, at least in the U.S., is largely concentrated on the realm of waived CLIA testing. As of October 2021, 68% of the POLs in the United States were primarily running CLIA waived tests.[6] CLIA test complexity has three levels: high, moderate, and waived.[7] Waived tests are simple to perform and have a relatively low risk of an incorrect test result. Moderately complex tests include tests like provider performed microscopy (PPM), which requires the use of a microscope during the office visit. Providers that want to perform PPM tests must be qualified to do so under CLIA regulations.[7] High-complexity tests require the most regulation. These tests are the most complicated and run the highest risk of an inaccurate result, as determined during the Food and Drug Administration (FDA) pre-market approval process. Tests may come from the manufacturer with their complexity level on them, or one can search the FDA database to determine the complexity of the test.[7]
Commonly performed tests include[8]:
- urine analysis
- urine pregnancy
- blood occult
- glucose blood
- pathology consultation during surgery
- crystal identification by microscope
- sperm identification and analyses
- bilirubin total
- blood gasses
- complete blood count
- bone marrow smear
- blood bank services
- transfusion medicine
References
- ↑ "Chapter 16 - Laboratory Services" (PDF). Medicare Claims Processing Manual. Centers for Medicare and Medicaid Services. 9 March 2021. https://www.cms.gov/Regulations-and-Guidance/Guidance/Manuals/downloads/clm104c16.pdf. Retrieved 18 November 2021.
- ↑ 2.0 2.1 "Physician Office Laboratory Evaluation Program (POLEP)". Wadsworth Center New York State Department of Health. https://www.wadsworth.org/regulatory/polep. Retrieved 18 November 2021.
- ↑ "Physician Office Laboratories or Clinics - Frequently Asked Questions about Clinical Laboratory Licensing and Registration" (PDF). California Department of Public Health. May 2008. Archived from the original on 29 December 2016. https://web.archive.org/web/20161229143212/http://www.cdph.ca.gov/programs/lfs/Documents/POL-FAQ.pdf. Retrieved 03 January 2020.
- ↑ "Laboratories by Type of Facility" (PDF). Centers for Medicare and Medicaid Services. October 2021. https://www.cms.gov/Regulations-and-Guidance/Legislation/CLIA/downloads/factype.pdf. Retrieved 18 November 2021.
- ↑ "How and Where IVD Will Find Growth in the Global POL Market – Part 2". Kalorama Information. November 2014. Archived from the original on 17 April 2015. https://web.archive.org/web/20150417204832/http://www.kaloramainformation.com/article/2014-11/How-and-Where-IVD-Will-Find-Growth-Global-POL-Market-%E2%80%93-Part-2. Retrieved 03 January 2020.
- ↑ Centers for Medicare and Medicaid Services, Division of Laboratory Services (October 2021). "Enrollment, CLIA exempt states, and certification of accreditation by organization" (PDF). https://www.cms.gov/Regulations-and-Guidance/Legislation/CLIA/Downloads/statupda.pdf. Retrieved 18 November 2021.
- ↑ 7.0 7.1 7.2 Centers for Disease Control and Prevention (6 August 2018). "Clinical Laboratory Improvement Amendments (CLIA): Test complexities". https://www.cdc.gov/clia/test-complexities.html. Retrieved 18 November 2021.
- ↑ UnitedHealthcare Oxford (1 January 2018). "UnitedHealthcare Oxford's in-office laboratory testing and procedures list" (PDF). https://www.oxhp.com/secure/policy/in_office_laboratory_testing_and_procedures_list.pdf. Retrieved 18 November 2021.