Template:Article of the week
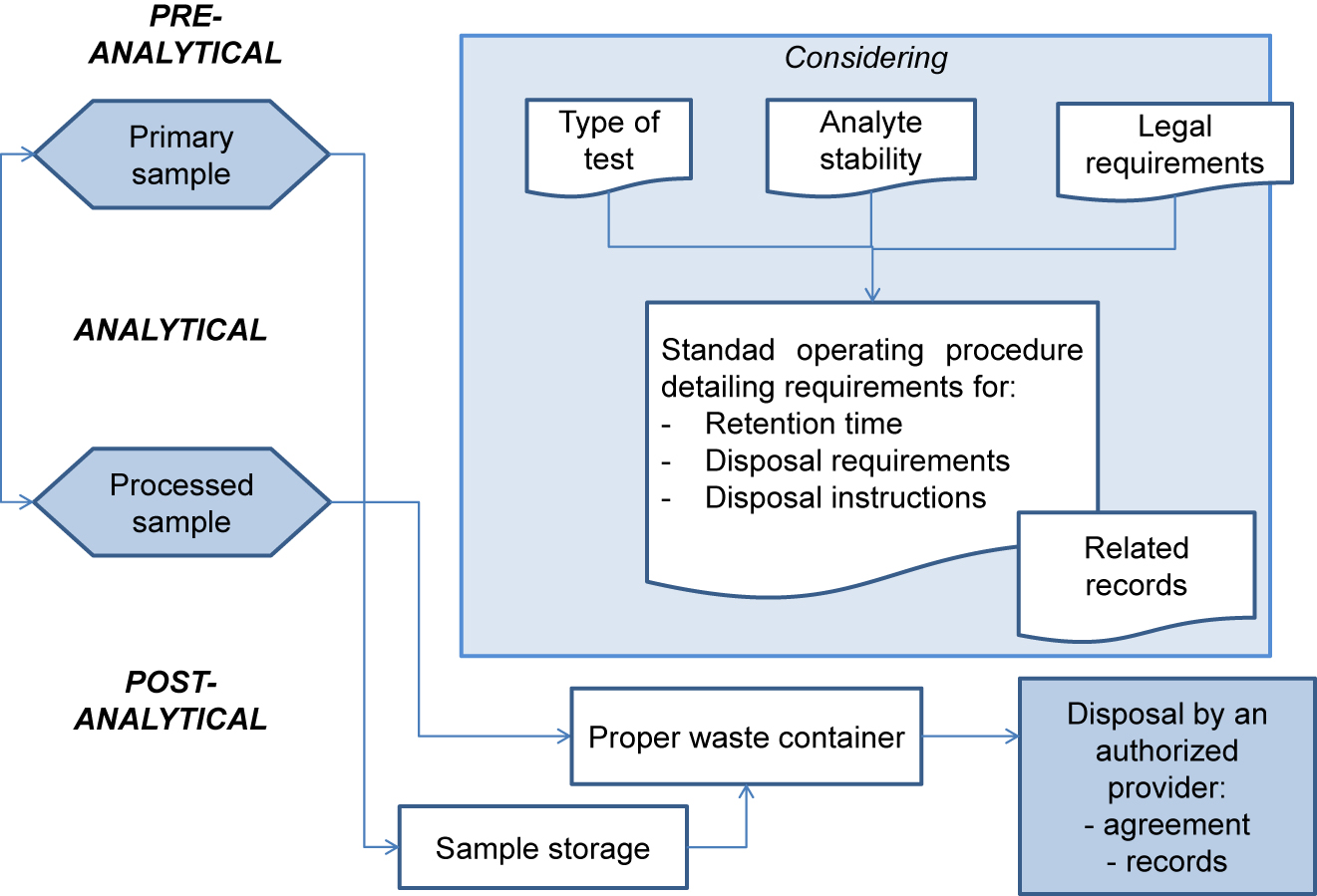
ISO 15189:2012 Medical laboratories — Requirements for quality and competence establishes the requirements for clinical specimen management, ensuring the quality of processes and laboratory information management. ENAC (Entidad Nacional de Acreditación), the sole accreditation authority in Spain, established the requirements for the authorized use of the ISO 15189 accreditation label in reports issued by accredited laboratories. These recommendations are applicable to the lab's post-analytical processes and the professionals involved. The standard requires laboratories to define and document the duration and conditions of specimen retention. Laboratories are also required to design an internal quality control scheme to verify whether post-analytical activities attain the expected standards. Information management requirements are also established, and laboratories are required to design a contingency plan to ensure the communication of laboratory results ... (Full article...)
Recently featured: