Difference between revisions of "Template:Article of the week"
From LIMSWiki
Jump to navigationJump to searchShawndouglas (talk | contribs) (Updated article of the week text.) |
Shawndouglas (talk | contribs) (Updated article of the week text) |
||
| Line 1: | Line 1: | ||
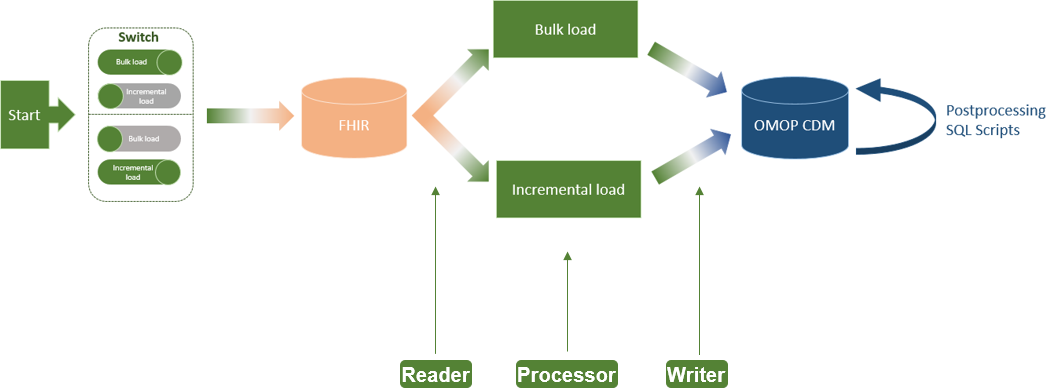
<div style="float: left; margin: 0.5em 0.9em 0.4em 0em;">[[File: | <div style="float: left; margin: 0.5em 0.9em 0.4em 0em;">[[File:Fig2 Henke JMIRMedInfo2023 11.png|240px]]</div> | ||
'''"[[Journal: | '''"[[Journal:An extract-transform-load process design for the incremental loading of German real-world data based on FHIR and OMOP CDM: Algorithm development and validation|An extract-transform-load process design for the incremental loading of German real-world data based on FHIR and OMOP CDM: Algorithm development and validation]]"''' | ||
In the Medical Informatics in Research and Care in University Medicine (MIRACUM) consortium, an IT-based clinical trial recruitment support system was developed based on the Observational Medical Outcomes Partnership (OMOP) Common Data Model (CDM). Currently, OMOP CDM is populated with German Fast Healthcare Interoperability Resources (FHIR) data using an extract-transform-load (ETL) process, which was designed as a bulk load. However, the computational effort that comes with an everyday full load is not efficient for daily recruitment ... ('''[[Journal:An extract-transform-load process design for the incremental loading of German real-world data based on FHIR and OMOP CDM: Algorithm development and validation|Full article...]]''')<br /> | |||
''Recently featured'': | ''Recently featured'': | ||
{{flowlist | | {{flowlist | | ||
* [[Journal:Potency and safety analysis of hemp-derived delta-9 products: The hemp vs. cannabis demarcation problem|Potency and safety analysis of hemp-derived delta-9 products: The hemp vs. cannabis demarcation problem]] | |||
* [[Journal:The NOMAD Artificial Intelligence Toolkit: Turning materials science data into knowledge and understanding|The NOMAD Artificial Intelligence Toolkit: Turning materials science data into knowledge and understanding]] | * [[Journal:The NOMAD Artificial Intelligence Toolkit: Turning materials science data into knowledge and understanding|The NOMAD Artificial Intelligence Toolkit: Turning materials science data into knowledge and understanding]] | ||
* [[Journal:Quality control in the clinical biochemistry laboratory: A glance|Quality control in the clinical biochemistry laboratory: A glance]] | * [[Journal:Quality control in the clinical biochemistry laboratory: A glance|Quality control in the clinical biochemistry laboratory: A glance]] | ||
}} | }} | ||
Revision as of 17:12, 20 February 2024
In the Medical Informatics in Research and Care in University Medicine (MIRACUM) consortium, an IT-based clinical trial recruitment support system was developed based on the Observational Medical Outcomes Partnership (OMOP) Common Data Model (CDM). Currently, OMOP CDM is populated with German Fast Healthcare Interoperability Resources (FHIR) data using an extract-transform-load (ETL) process, which was designed as a bulk load. However, the computational effort that comes with an everyday full load is not efficient for daily recruitment ... (Full article...)
Recently featured: