Difference between revisions of "Template:Article of the week"
Shawndouglas (talk | contribs) (Updated article of the week text) |
Shawndouglas (talk | contribs) (Updated article of the week text) |
||
| Line 1: | Line 1: | ||
<div style="float: left; margin: 0.5em 0.9em 0.4em 0em;">[[File:Fig1 | <div style="float: left; margin: 0.5em 0.9em 0.4em 0em;">[[File:Fig1 Naphade JofClinDiagRes2023 17-2.jpg|240px]]</div> | ||
'''"[[Journal: | '''"[[Journal:Quality control in the clinical biochemistry laboratory: A glance|Quality control in the clinical biochemistry laboratory: A glance]]"''' | ||
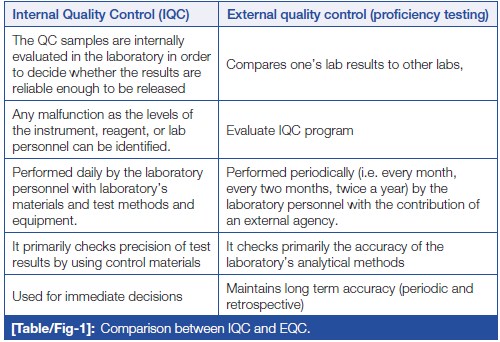
[Quality control]] (QC) is a process, designed to ensure reliable test results. It is part of overall [[laboratory]] quality management in terms of accuracy, reliability, and timeliness of reported test results. Two types of QC are exercised in [[Clinical chemistry|clinical biochemistry]]: internal QC (IQC) and external [[quality assurance]] (QA). IQC represents the quality methods performed every day by laboratory personnel with the laboratory’s materials and equipment. It primarily checks the precision (i.e., repeatability or reproducibility) of the test method. External quality assurance service (EQAS) is performed periodically (i.e., every month, every two months, twice a year) by the laboratory personnel, who primarily are checking the accuracy of the laboratory’s analytical methods ... ('''[[Journal:Quality control in the clinical biochemistry laboratory: A glance|Full article...]]''')<br /> | |||
''Recently featured'': | ''Recently featured'': | ||
{{flowlist | | {{flowlist | | ||
* [[Journal:Shared metadata for data-centric materials science|Shared metadata for data-centric materials science]] | |||
* [[Journal:A metabolomics and big data approach to cannabis authenticity (authentomics)|A metabolomics and big data approach to cannabis authenticity (authentomics)]] | * [[Journal:A metabolomics and big data approach to cannabis authenticity (authentomics)|A metabolomics and big data approach to cannabis authenticity (authentomics)]] | ||
* [[Journal:Integration of X-ray absorption fine structure databases for data-driven materials science|Integration of X-ray absorption fine structure databases for data-driven materials science]] | * [[Journal:Integration of X-ray absorption fine structure databases for data-driven materials science|Integration of X-ray absorption fine structure databases for data-driven materials science]] | ||
}} | }} | ||
Revision as of 18:39, 29 January 2024
"Quality control in the clinical biochemistry laboratory: A glance"
[Quality control]] (QC) is a process, designed to ensure reliable test results. It is part of overall laboratory quality management in terms of accuracy, reliability, and timeliness of reported test results. Two types of QC are exercised in clinical biochemistry: internal QC (IQC) and external quality assurance (QA). IQC represents the quality methods performed every day by laboratory personnel with the laboratory’s materials and equipment. It primarily checks the precision (i.e., repeatability or reproducibility) of the test method. External quality assurance service (EQAS) is performed periodically (i.e., every month, every two months, twice a year) by the laboratory personnel, who primarily are checking the accuracy of the laboratory’s analytical methods ... (Full article...)
Recently featured: