Difference between revisions of "Template:Article of the week"
Shawndouglas (talk | contribs) |
Shawndouglas (talk | contribs) (Updated article of the week text) |
||
| Line 1: | Line 1: | ||
<div style="float: left; margin: 0.5em 0.9em 0.4em 0em;">[[File:Fig1 | <div style="float: left; margin: 0.5em 0.9em 0.4em 0em;">[[File:Fig1 Heavey ForSciIntSyn2023 7.jpg|240px]]</div> | ||
'''"[[Journal: | '''"[[Journal:Management and disclosure of quality issues in forensic science: A survey of current practice in Australia and New Zealand|Management and disclosure of quality issues in forensic science: A survey of current practice in Australia and New Zealand]]"''' | ||
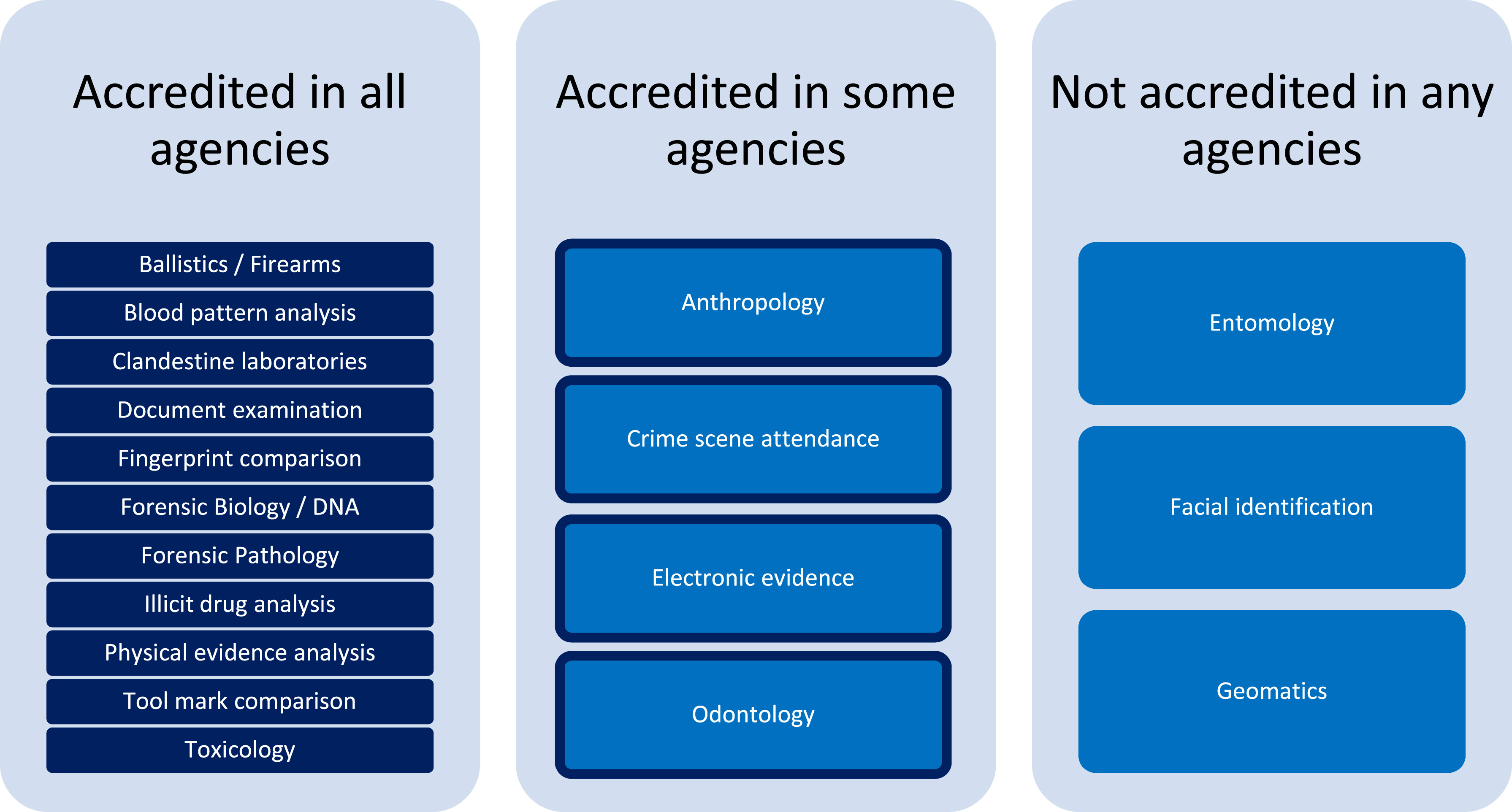
[[ | The investigation of [[Quality (business)|quality]] issues detected within the [[Forensic science|forensic]] process is a critical feature in robust [[quality management system]]s (QMSs) to provide assurance of the validity of reported [[laboratory]] results and inform strategies for [[Continual improvement process|continuous improvement]] and innovation. A survey was conducted to gain insight into the current state of practice in the management and handling of quality issues amongst the government service provider agencies of Australia and New Zealand. The results demonstrate the value of standardized quality system structures for the recording and management of quality issues, but also areas where inconsistent reporting increases the risk of overlooking important data to inform continuous improvement ... ('''[[Journal:Management and disclosure of quality issues in forensic science: A survey of current practice in Australia and New Zealand|Full article...]]''')<br /> | ||
''Recently featured'': | ''Recently featured'': | ||
{{flowlist | | {{flowlist | | ||
* [[Journal:Thirty years of the DICOM standard|Thirty years of the DICOM standard]] | |||
* [[Journal:Establishing reliable research data management by integrating measurement devices utilizing intelligent digital twins|Establishing reliable research data management by integrating measurement devices utilizing intelligent digital twins]] | |||
* [[Journal:Evaluating the effectiveness of a new student-centred laboratory training strategy in clinical biochemistry teaching|Evaluating the effectiveness of a new student-centred laboratory training strategy in clinical biochemistry teaching]] | * [[Journal:Evaluating the effectiveness of a new student-centred laboratory training strategy in clinical biochemistry teaching|Evaluating the effectiveness of a new student-centred laboratory training strategy in clinical biochemistry teaching]] | ||
}} | }} | ||
Revision as of 15:44, 2 January 2024
The investigation of quality issues detected within the forensic process is a critical feature in robust quality management systems (QMSs) to provide assurance of the validity of reported laboratory results and inform strategies for continuous improvement and innovation. A survey was conducted to gain insight into the current state of practice in the management and handling of quality issues amongst the government service provider agencies of Australia and New Zealand. The results demonstrate the value of standardized quality system structures for the recording and management of quality issues, but also areas where inconsistent reporting increases the risk of overlooking important data to inform continuous improvement ... (Full article...)
Recently featured: