Difference between revisions of "Template:Article of the week"
From LIMSWiki
Jump to navigationJump to searchShawndouglas (talk | contribs) (Updated article of the week text) |
Shawndouglas (talk | contribs) (Updated article of the week text) |
||
| Line 1: | Line 1: | ||
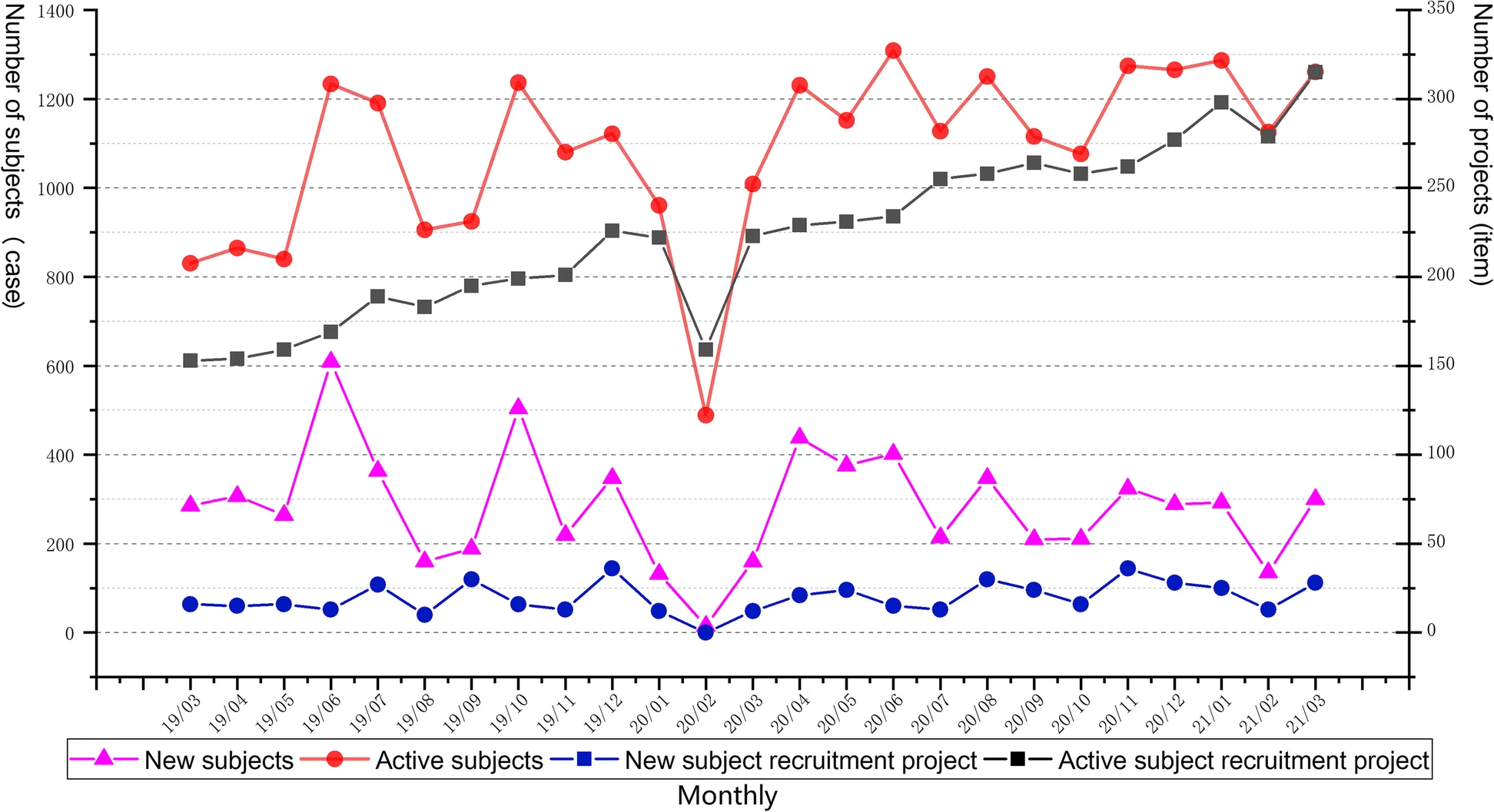
<div style="float: left; margin: 0.5em 0.9em 0.4em 0em;">[[File: | <div style="float: left; margin: 0.5em 0.9em 0.4em 0em;">[[File:Fig4 Shen BMCMedInfoDecMak23 23.png|240px]]</div> | ||
'''"[[Journal: | '''"[[Journal:Development of an integrated and comprehensive clinical trial process management system|Development of an integrated and comprehensive clinical trial process management system]]"''' | ||
[[ | The process of initiating and completing clinical drug trials in [[hospital]] settings is highly complex, with numerous institutional, technical, and record-keeping barriers. In this study, we independently developed an integrated [[clinical trial management system]] (CTMS) designed to comprehensively optimize the process management of clinical trials. The CTMS includes system development methods, efficient integration with external business systems, terminology, and standardization protocols, as well as [[Information security|data security]] and [[Information privacy|privacy]] protection ... ('''[[Journal:Development of an integrated and comprehensive clinical trial process management system|Full article...]]''')<br /> | ||
''Recently featured'': | ''Recently featured'': | ||
{{flowlist | | {{flowlist | | ||
* [[Journal:A web application to support the coordination of reflexive, interpretative toxicology testing|A web application to support the coordination of reflexive, interpretative toxicology testing]] | |||
* [[Journal:ApE, A Plasmid Editor: A freely available DNA manipulation and visualization program|ApE, A Plasmid Editor: A freely available DNA manipulation and visualization program]] | * [[Journal:ApE, A Plasmid Editor: A freely available DNA manipulation and visualization program|ApE, A Plasmid Editor: A freely available DNA manipulation and visualization program]] | ||
* [[Journal:Development and national scale implementation of an open-source electronic laboratory information system (OpenELIS) in Côte d’Ivoire: Sustainability lessons from the first 13 years|Development and national scale implementation of an open-source electronic laboratory information system (OpenELIS) in Côte d’Ivoire: Sustainability lessons from the first 13 years]] | * [[Journal:Development and national scale implementation of an open-source electronic laboratory information system (OpenELIS) in Côte d’Ivoire: Sustainability lessons from the first 13 years|Development and national scale implementation of an open-source electronic laboratory information system (OpenELIS) in Côte d’Ivoire: Sustainability lessons from the first 13 years]] | ||
}} | }} | ||
Revision as of 17:17, 16 October 2023
"Development of an integrated and comprehensive clinical trial process management system"
The process of initiating and completing clinical drug trials in hospital settings is highly complex, with numerous institutional, technical, and record-keeping barriers. In this study, we independently developed an integrated clinical trial management system (CTMS) designed to comprehensively optimize the process management of clinical trials. The CTMS includes system development methods, efficient integration with external business systems, terminology, and standardization protocols, as well as data security and privacy protection ... (Full article...)
Recently featured:
- A web application to support the coordination of reflexive, interpretative toxicology testing
- ApE, A Plasmid Editor: A freely available DNA manipulation and visualization program
- Development and national scale implementation of an open-source electronic laboratory information system (OpenELIS) in Côte d’Ivoire: Sustainability lessons from the first 13 years