Difference between revisions of "Template:Article of the week"
Shawndouglas (talk | contribs) (Added last week's article of the week) |
Shawndouglas (talk | contribs) (Updated article of the week text) |
||
| Line 1: | Line 1: | ||
<div style="float: left; margin: 0.5em 0.9em 0.4em 0em;">[[File: | <div style="float: left; margin: 0.5em 0.9em 0.4em 0em;">[[File:Fig1 Miguel QuimicaNova22 44-6.png|240px]]</div> | ||
'''"[[Journal: | '''"[[Journal:ISO/IEC 17025: History and introduction of concepts|ISO/IEC 17025: History and introduction of concepts]]"''' | ||
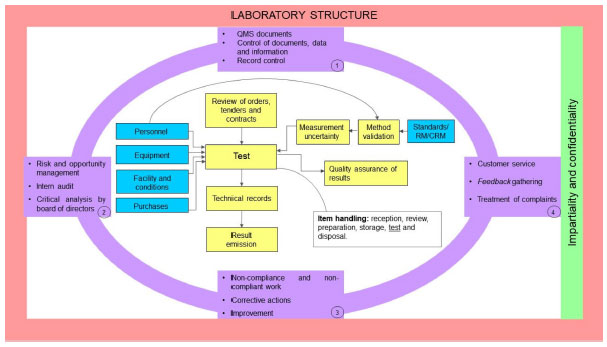
[[Quality (business)|Quality]] is an increasingly present concept nowadays, and meeting the needs of customers who buy and use products and hire services becomes essential. For [[Laboratory|laboratories]], the concept is applied not only to the reliability and traceability of the results produced, but it also presents itself in meeting the customer’s needs and providing confidence when signing agreements in the international trade. The concept of quality in a laboratory can be carried out from the development and implementation of a [[quality management system]] (QMS). To this end, the normative, internationally accepted document [[ISO/IEC 17025]] aims at instructing the development and implementation of a management system, which ideally proves the technical capacity of testing and [[Reference laboratory|calibration laboratories]] and guides the generation of reliable results ... ('''[[Journal:ISO/IEC 17025: History and introduction of concepts|Full article...]]''')<br /> | |||
''Recently featured'': | ''Recently featured'': | ||
{{flowlist | | {{flowlist | | ||
* [[Journal:Practical considerations for laboratories: Implementing a holistic quality management system|Practical considerations for laboratories: Implementing a holistic quality management system]] | |||
* [[Journal:Precision nutrition: Maintaining scientific integrity while realizing market potential|Precision nutrition: Maintaining scientific integrity while realizing market potential]] | * [[Journal:Precision nutrition: Maintaining scientific integrity while realizing market potential|Precision nutrition: Maintaining scientific integrity while realizing market potential]] | ||
* [[Journal:Construction of control charts to help in the stability and reliability of results in an accredited water quality control laboratory|Construction of control charts to help in the stability and reliability of results in an accredited water quality control laboratory]] | * [[Journal:Construction of control charts to help in the stability and reliability of results in an accredited water quality control laboratory|Construction of control charts to help in the stability and reliability of results in an accredited water quality control laboratory]] | ||
}} | }} | ||
Revision as of 15:07, 5 June 2023
"ISO/IEC 17025: History and introduction of concepts"
Quality is an increasingly present concept nowadays, and meeting the needs of customers who buy and use products and hire services becomes essential. For laboratories, the concept is applied not only to the reliability and traceability of the results produced, but it also presents itself in meeting the customer’s needs and providing confidence when signing agreements in the international trade. The concept of quality in a laboratory can be carried out from the development and implementation of a quality management system (QMS). To this end, the normative, internationally accepted document ISO/IEC 17025 aims at instructing the development and implementation of a management system, which ideally proves the technical capacity of testing and calibration laboratories and guides the generation of reliable results ... (Full article...)
Recently featured:
- Practical considerations for laboratories: Implementing a holistic quality management system
- Precision nutrition: Maintaining scientific integrity while realizing market potential
- Construction of control charts to help in the stability and reliability of results in an accredited water quality control laboratory