Difference between revisions of "Template:Article of the week"
Shawndouglas (talk | contribs) (Updated article of the week text.) |
Shawndouglas (talk | contribs) (Updated article of the week text) |
||
| Line 1: | Line 1: | ||
<div style="float: left; margin: 0.5em 0.9em 0.4em 0em;">[[File: | <div style="float: left; margin: 0.5em 0.9em 0.4em 0em;">[[File:Fig2 Yoon LabAniRes22 38.png|240px]]</div> | ||
'''"[[Journal: | '''"[[Journal:Laboratory information management system for COVID-19 non-clinical efficacy trial data|Laboratory information management system for COVID-19 non-clinical efficacy trial data]]"''' | ||
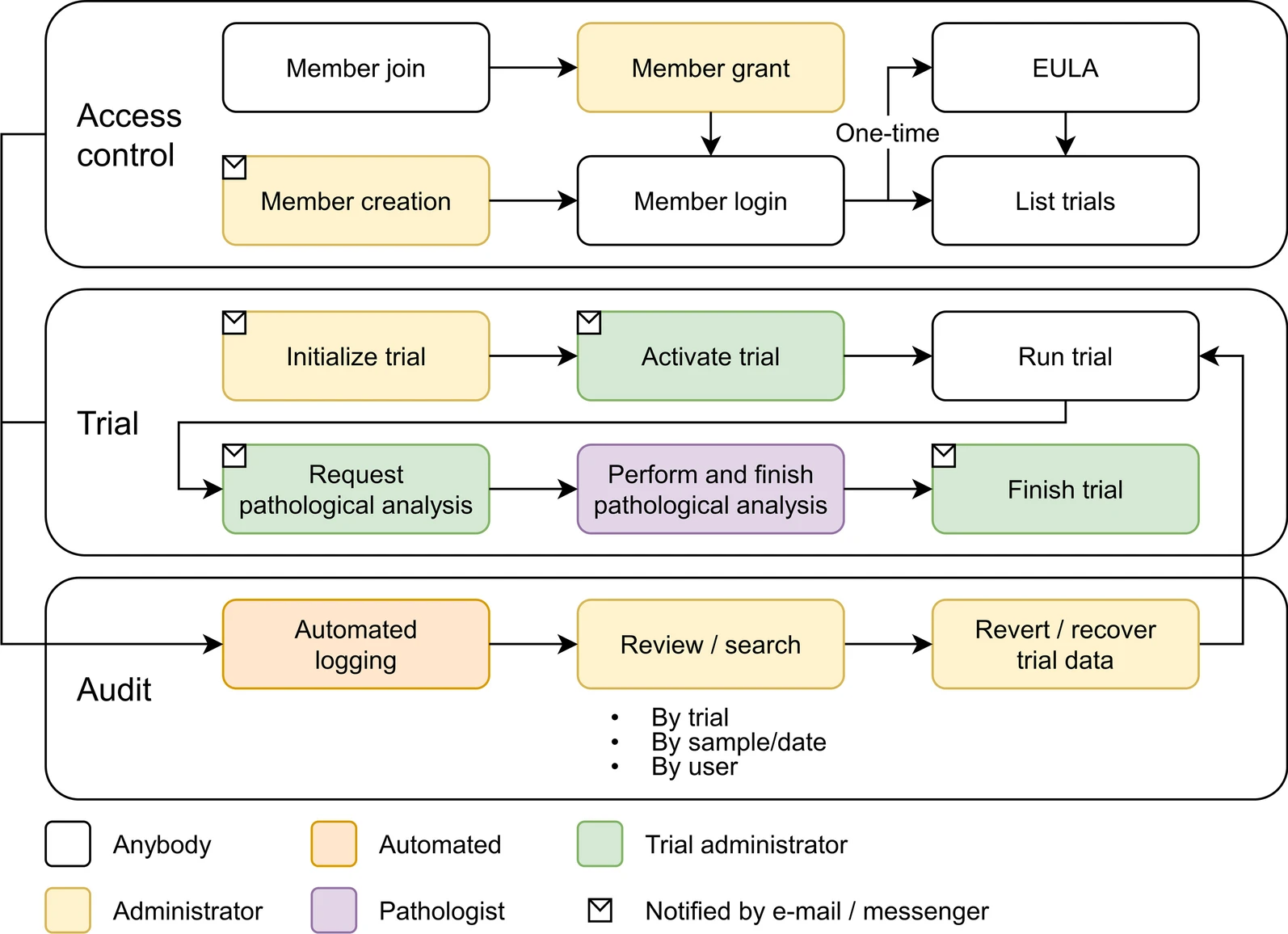
As the number of large-scale research studies involving multiple organizations producing data has steadily increased, an integrated system for a common interoperable data format is needed. For example, in response to the [[coronavirus disease 2019]] (COVID-19) [[pandemic]], a number of global efforts are underway to develop vaccines and therapeutics. We are therefore observing an explosion in the proliferation of COVID-19 data, and interoperability is highly requested in multiple institutions participating simultaneously in COVID-19 pandemic research. In this study, a [[laboratory information management system]] (LIMS) has been adopted to systemically manage, via web interface, various COVID-19 non-clinical trial data—including mortality, clinical signs, body weight, body temperature, organ weights, viral titer (viral replication and viral RNA), and multi-organ [[histopathology]]—from multiple institutions ... ('''[[Journal:Laboratory information management system for COVID-19 non-clinical efficacy trial data|Full article...]]''')<br /> | |||
<br /> | <br /> | ||
''Recently featured'': | ''Recently featured'': | ||
{{flowlist | | {{flowlist | | ||
* [[Journal:Improving data quality in clinical research informatics tools|Improving data quality in clinical research informatics tools]] | |||
* [[Journal:Electronic tools in clinical laboratory diagnostics: Key examples, limitations, and value in laboratory medicine|Electronic tools in clinical laboratory diagnostics: Key examples, limitations, and value in laboratory medicine]] | * [[Journal:Electronic tools in clinical laboratory diagnostics: Key examples, limitations, and value in laboratory medicine|Electronic tools in clinical laboratory diagnostics: Key examples, limitations, and value in laboratory medicine]] | ||
* [[Journal:Anatomic pathology quality assurance: Developing an LIS-based tracking and documentation module for intradepartmental consultations|Anatomic pathology quality assurance: Developing an LIS-based tracking and documentation module for intradepartmental consultations]] | * [[Journal:Anatomic pathology quality assurance: Developing an LIS-based tracking and documentation module for intradepartmental consultations|Anatomic pathology quality assurance: Developing an LIS-based tracking and documentation module for intradepartmental consultations]] | ||
}} | }} | ||
Revision as of 19:43, 21 February 2023
"Laboratory information management system for COVID-19 non-clinical efficacy trial data"
As the number of large-scale research studies involving multiple organizations producing data has steadily increased, an integrated system for a common interoperable data format is needed. For example, in response to the coronavirus disease 2019 (COVID-19) pandemic, a number of global efforts are underway to develop vaccines and therapeutics. We are therefore observing an explosion in the proliferation of COVID-19 data, and interoperability is highly requested in multiple institutions participating simultaneously in COVID-19 pandemic research. In this study, a laboratory information management system (LIMS) has been adopted to systemically manage, via web interface, various COVID-19 non-clinical trial data—including mortality, clinical signs, body weight, body temperature, organ weights, viral titer (viral replication and viral RNA), and multi-organ histopathology—from multiple institutions ... (Full article...)
Recently featured:
- Improving data quality in clinical research informatics tools
- Electronic tools in clinical laboratory diagnostics: Key examples, limitations, and value in laboratory medicine
- Anatomic pathology quality assurance: Developing an LIS-based tracking and documentation module for intradepartmental consultations