Difference between revisions of "Template:Article of the week"
From LIMSWiki
Jump to navigationJump to searchShawndouglas (talk | contribs) (Updated article of the week text) |
Shawndouglas (talk | contribs) (Updated article of the week text.) |
||
| Line 1: | Line 1: | ||
<div style="float: left; margin: 0.5em 0.9em 0.4em 0em;">[[File:Fig1 | <div style="float: left; margin: 0.5em 0.9em 0.4em 0em;">[[File:Fig1 AbuHalimeh FrontBigData2022 5.jpg|240px]]</div> | ||
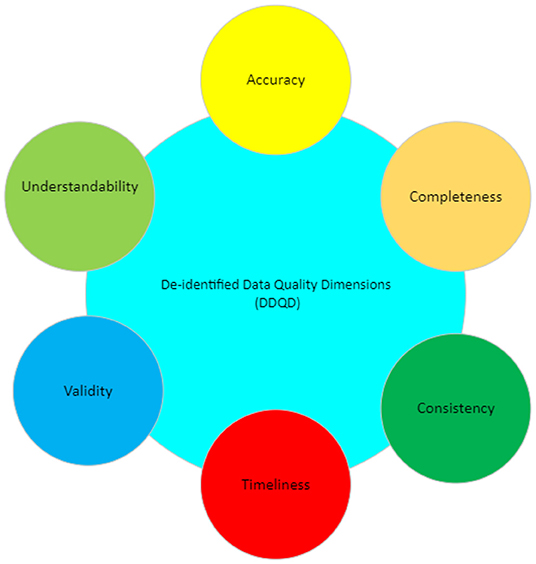
'''"[[Journal: | '''"[[Journal:Improving data quality in clinical research informatics tools|Improving data quality in clinical research informatics tools]]"''' | ||
Maintaining [[data quality]] is a fundamental requirement for any successful and long-term [[Information management|data management]] project. Providing high-quality, reliable, and statistically sound data is a primary goal for [[wikipedia:Clinical research|clinical research]] [[Informatics (academic field)|informatics]]. In addition, effective data governance and management are essential to ensuring accurate data counts, reports, and validation. As a crucial step of the clinical research process, it is important to establish and maintain organization-wide standards for data quality management to ensure consistency across all systems designed primarily for cohort identification ... ('''[[Journal:Improving data quality in clinical research informatics tools|Full article...]]''')<br /> | |||
<br /> | <br /> | ||
''Recently featured'': | ''Recently featured'': | ||
{{flowlist | | {{flowlist | | ||
* [[Journal:Electronic tools in clinical laboratory diagnostics: Key examples, limitations, and value in laboratory medicine|Electronic tools in clinical laboratory diagnostics: Key examples, limitations, and value in laboratory medicine]] | |||
* [[Journal:Anatomic pathology quality assurance: Developing an LIS-based tracking and documentation module for intradepartmental consultations|Anatomic pathology quality assurance: Developing an LIS-based tracking and documentation module for intradepartmental consultations]] | * [[Journal:Anatomic pathology quality assurance: Developing an LIS-based tracking and documentation module for intradepartmental consultations|Anatomic pathology quality assurance: Developing an LIS-based tracking and documentation module for intradepartmental consultations]] | ||
* [[Journal:Using knowledge graph structures for semantic interoperability in electronic health records data exchanges|Using knowledge graph structures for semantic interoperability in electronic health records data exchanges]] | * [[Journal:Using knowledge graph structures for semantic interoperability in electronic health records data exchanges|Using knowledge graph structures for semantic interoperability in electronic health records data exchanges]] | ||
}} | }} | ||
Revision as of 16:37, 13 February 2023
"Improving data quality in clinical research informatics tools"
Maintaining data quality is a fundamental requirement for any successful and long-term data management project. Providing high-quality, reliable, and statistically sound data is a primary goal for clinical research informatics. In addition, effective data governance and management are essential to ensuring accurate data counts, reports, and validation. As a crucial step of the clinical research process, it is important to establish and maintain organization-wide standards for data quality management to ensure consistency across all systems designed primarily for cohort identification ... (Full article...)
Recently featured:
- Electronic tools in clinical laboratory diagnostics: Key examples, limitations, and value in laboratory medicine
- Anatomic pathology quality assurance: Developing an LIS-based tracking and documentation module for intradepartmental consultations
- Using knowledge graph structures for semantic interoperability in electronic health records data exchanges