Difference between revisions of "Template:Article of the week"
Shawndouglas (talk | contribs) (Updated article of the week text) |
Shawndouglas (talk | contribs) (Updated article of the week text) |
||
| Line 1: | Line 1: | ||
<div style="float: left; margin: 0.5em 0.9em 0.4em 0em;">[[File:Fig2 | <div style="float: left; margin: 0.5em 0.9em 0.4em 0em;">[[File:Fig2 Starks JPathInfo2021 12.jpg|120px]]</div> | ||
'''"[[Journal: | '''"[[Journal:Use of middleware data to dissect and optimize hematology autoverification|Use of middleware data to dissect and optimize hematology autoverification]]"''' | ||
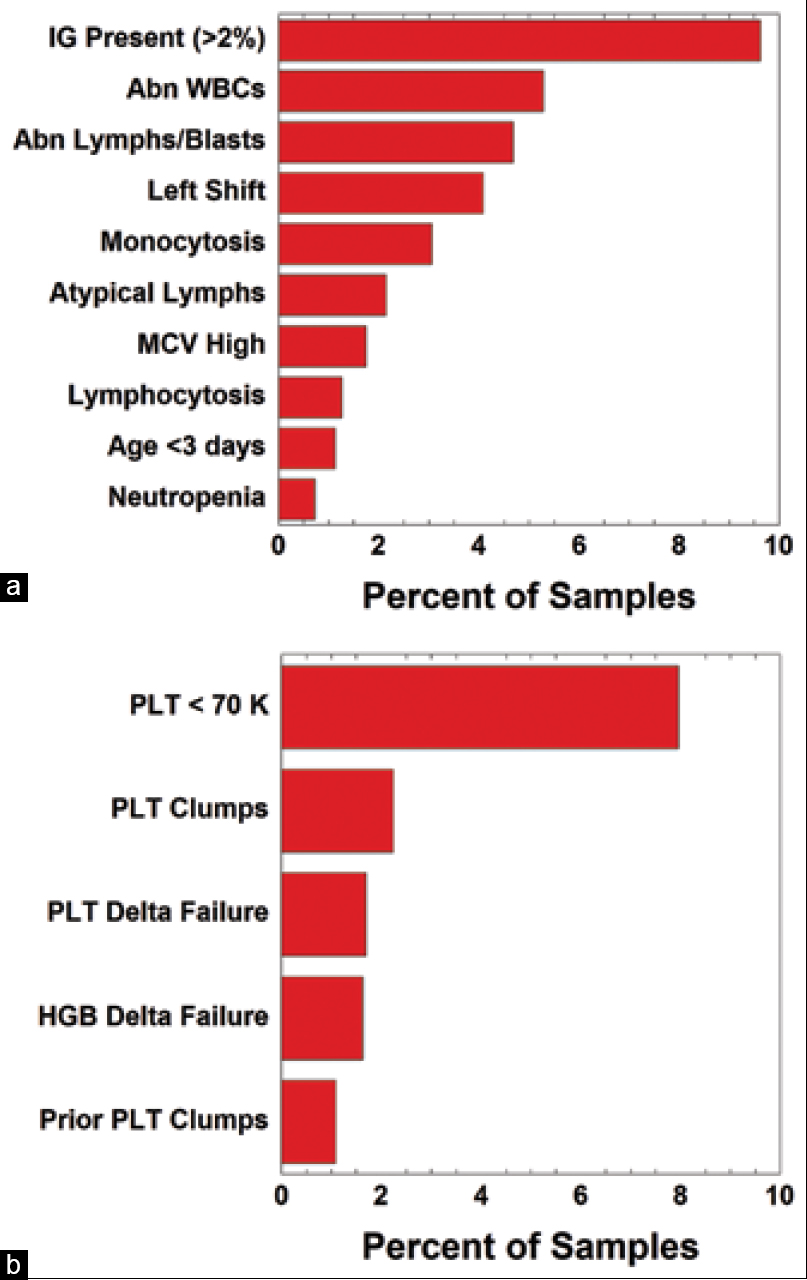
[[Hematology]] [[analysis]] comprises some of the highest volume tests run in [[Clinical laboratory|clinical laboratories]]. Autoverification of hematology results using computer-based rules reduces turnaround time for many [[Sample (material)|specimens]], while strategically targeting specimen review by technologist or pathologist. Autoverification rules had been developed over a decade at an 800-bed tertiary/quarternary care academic medical central laboratory serving both adult and pediatric populations. In the process of migrating to newer hematology instruments, we analyzed the rates of the autoverification rules/flags most commonly associated with triggering manual review. We were particularly interested in rules that on their own often led to manual review in the absence of other flags. Prior to the study, autoverification rates were 87.8% (out of 16,073 orders) for complete blood count (CBC) if ordered as a panel and 85.8% (out of 1,940 orders) for CBC components ordered individually (not as the panel). ('''[[Journal:Use of middleware data to dissect and optimize hematology autoverification|Full article...]]''')<br /> | |||
<br /> | <br /> | ||
''Recently featured'': | ''Recently featured'': | ||
{{flowlist | | {{flowlist | | ||
* [[Journal:Automated cyber and privacy risk management toolkit|Automated cyber and privacy risk management toolkit]] | |||
* [[Journal:Design of generalized search interfaces for health informatics|Design of generalized search interfaces for health informatics]] | * [[Journal:Design of generalized search interfaces for health informatics|Design of generalized search interfaces for health informatics]] | ||
* [[Journal:Cybersecurity and privacy risk assessment of point-of-care systems in healthcare: A use case approach|Cybersecurity and privacy risk assessment of point-of-care systems in healthcare: A use case approach]] | * [[Journal:Cybersecurity and privacy risk assessment of point-of-care systems in healthcare: A use case approach|Cybersecurity and privacy risk assessment of point-of-care systems in healthcare: A use case approach]] | ||
}} | }} | ||
Revision as of 20:08, 12 June 2022
"Use of middleware data to dissect and optimize hematology autoverification"
Hematology analysis comprises some of the highest volume tests run in clinical laboratories. Autoverification of hematology results using computer-based rules reduces turnaround time for many specimens, while strategically targeting specimen review by technologist or pathologist. Autoverification rules had been developed over a decade at an 800-bed tertiary/quarternary care academic medical central laboratory serving both adult and pediatric populations. In the process of migrating to newer hematology instruments, we analyzed the rates of the autoverification rules/flags most commonly associated with triggering manual review. We were particularly interested in rules that on their own often led to manual review in the absence of other flags. Prior to the study, autoverification rates were 87.8% (out of 16,073 orders) for complete blood count (CBC) if ordered as a panel and 85.8% (out of 1,940 orders) for CBC components ordered individually (not as the panel). (Full article...)
Recently featured: