Difference between revisions of "Journal:Digitalization concepts in academic bioprocess development"
Shawndouglas (talk | contribs) (Saving and adding more.) |
Shawndouglas (talk | contribs) |
||
| Line 97: | Line 97: | ||
|} | |} | ||
|} | |} | ||
==Digitalization strategies== | |||
==Abbreviations, acronyms, and initialisms== | ==Abbreviations, acronyms, and initialisms== | ||
Revision as of 03:06, 23 April 2024
| Full article title | Digitalization concepts in academic bioprocess development |
|---|---|
| Journal | Engineering in Life Sciences |
| Author(s) | Habich, Tessa; Beutel, Sascha |
| Author affiliation(s) | Leibniz University Hannover |
| Primary contact | Email: beutel at iftc dot uni dash hannover dot de |
| Year published | 2024 |
| Volume and issue | 24(4) |
| Article # | 2300238 |
| DOI | 10.1002/elsc.202300238 |
| ISSN | 1618-2863 |
| Distribution license | Creative Commons Attribution-NonCommercial 4.0 International |
| Website | https://analyticalsciencejournals.onlinelibrary.wiley.com/doi/10.1002/elsc.202300238 |
| Download | https://analyticalsciencejournals.onlinelibrary.wiley.com/doi/pdfdirect/10.1002/elsc.202300238 (PDF) |
|
|
This article should be considered a work in progress and incomplete. Consider this article incomplete until this notice is removed. |
Abstract
Digitalization with integrated devices, digital and physical assistants, automation, and simulation is setting a new direction for laboratory work. Even with complex research workflows, high staff turnover, and a limited budget, some laboratories have already shown that digitalization is indeed possible. However, academic bioprocess laboratories often struggle to follow the trend of digitalization. Due to their diverse research circumstances, high variety of team composition, goals, and limitations, the concepts are substantially different. Here, we will provide an overview on different aspects of digitalization and describe how academic research laboratories successfully digitalized their working environment. The key aspect is the collaboration and communication between IT-experts and scientific staff. The developed digital infrastructure is only useful if it supports the laboratory worker and does not complicate their work. Thereby, laboratory researchers have to collaborate closely with IT experts in order for a well-developed and maintainable digitalization concept that fits their individual needs and level of complexity to emerge. This review may serve as a starting point or a collection of ideas for the transformation toward a digitalized bioprocess laboratory.
Keywords: academic laboratories, automation, bioprocess, digitalization, FAIR data, Laboratory and Analytical Device Standard (LADS), Standardization in Lab Automation 2 (SiLA2)
Introduction
In contrast to industry, academic research laboratories require more flexibility than production lines. Besides the need for flexibility, loss of knowledge due to high staff turnover in universities is another challenge that makes full laboratory digitalization hard to achieve. [1-4] "Digitalization" (what we'll be addressing) refers to an entire process, workflow, or laboratory infrastructure, whereas "digitization" refers only to the procedure of converting something analog to a digital format (e.g., digitizing a standard operating procedure [SOP] from a piece of paper to a digital file). [5, 6] Working in digital laboratories has the potential for error reduction, prevention of data loss, improved data integrity, faster workflow development times, possible reduction of chemical and material use, and higher sample throughput, leading to modern, transparent, and reproducible research and biomanufacturing. [7-14]
Developing a digitalization strategy for an academic bioprocess laboratory is an interdisciplinary task. Laboratory workers have to work closely with IT experts in order to achieve a digital infrastructure that can be maintained and is supporting their laboratory work rather than hindering it. [5, 7, 9, 15, 16] This kind of collaboration is rarely found in academic bioprocess laboratories, so even the smallest digitalization task can be a major challenge. Hardware vendors selling their devices with proprietary software and restrictive access and information about their interfaces is making digitalization even more difficult. [1, 7, 17] Therefore, academic researchers working in the field of digitalization are developing concepts that are fitting their individual needs at the time. This is why there are manifold digitalization concepts throughout academic laboratories.
This review will present different digitalization concepts throughout academic bioprocess development laboratories. Before looking at different individual concepts in more detail, general aspects like FAIR data (data that is readily findable, accessible, interoperable, and reusable), standard communication protocols, digital twins, and interaction with laboratory devices will be addressed.
Basic concepts of digitalization
FAIR data
With the progressing digitalization of laboratories, the generated amount of data is steadily increasing. Therefore, good data management systems will become inevitable in bioprocess development laboratories. The first step towards good data management is storing the data and metadata according to the FAIR data principles. [18] Both humans and machines should be able to find the data with metadata and a clear unique identifier. The data should be digitally accessible for the user with the appropriate tool. It should be noted that accessible in this context does not mean that the data are "open" or "free" but that transparency around the used data concerning its availability is given. [19, 20] Interoperable data means data are presented using vocabulary and data encoding that follows the FAIR data principles and can be read by machines. In order for data to be reusable they need to be rich in metadata and descriptive documentation on the circumstances in which the data were generated. Rich metadata should ideally describe the data in a meaningful way, including the setup and context of the experiment, technical setting, and information about the provenance. [8, 18, 21–23]
Most research data are currently stored locally and not organized in a standardized way. Storing data according to FAIR data principles allows them to be read by machines and not just the human laboratory worker who generated them. Other advantages of following the FAIR data principles, as well as publishing research data, is that this data will stay available over time. Available or even openly online shared data, including metadata, can help to determine if the publication is of high quality. It can be reproduced, reanalyzed, used for new analyses, or even compared to or combined with other data promoting a deeper understanding of the topic or perhaps generating new knowledge. [12, 21, 24]
Before computers were used on a large scale in laboratories, results of experiments were handwritten into paper notebooks. This is still happening in many cases in today's bioprocess development laboratories, although the use of electronic laboratory notebooks (ELNs) is emerging quickly. [25] Most devices are equipped with a USB, RS232, or RS485 port, thus providing network connectivity. But the absence of a physical network necessitates the usage of, for example, flash drives for data transport. This increases the danger of manipulation or loss of data. [9] Writing down data manually and then digitizing it later on a computer used for data storage and analysis is also not a good solution. The analog handwritten data is prone to error, not good to search through, only accessible from the analog laboratory notebook, and has limited reusability, in contrast to the FAIR data principles. [9, 26] After it has been digitized without human errors, it has all the advantages of digital data. In order to make data gathering faster, simpler, and more accurate, a complete transition to automated data capture has to be made. [26] This includes data flow from a whole bioprocess, including sampling, sample preparation, measurement, and data analysis. [8] When transitioning from analog to digital data, it is important to take the laboratory users needs into consideration. While aiming to automatically store data following FAIR data principles, the end user who is analyzing it still needs to be able to access and work with it.
Standard communication protocols
While a lot of laboratory device vendors still offer their own proprietary software for device control [17, 27], researchers are agreeing that the future of digitalization in the laboratory lies in the common use of standardized device communication protocols. [7, 9, 17, 26, 28–34] To connect all laboratory devices to one digital infrastructure, it is aspirational for all devices to use the same standard communication protocol to achieve a plug-and-play environment in bioprocess laboratories. [26] Device integration is faster, easier, and better to maintain when vendor-independent communication protocols are more common. [35]
While offering device control with standard communication protocols might be costly for vendors in the beginning due to initial software development, it offers advantages as well. Using a communication standard as an instrument vendor will reduce the complexity of their software documentation since the basics have already been well documented. Thus, a detailed documentation of manufacturer-specific proprietary device drivers will become redundant. This simplification of software development can then reduce costs for device vendors. When using standard protocols for device communication, instrument vendors are contributing to the management of data according to the FAIR data principles, leaving them to focus on new innovations and features in their devices. Customers can then choose devices based upon those innovations instead of technical practicalities. Additionally, more companies and laboratories have the ability to integrate devices with standard communication protocols into their digital infrastructures because they are not limited by interface or platform incompatibilities. This will then lead to an increased purchase of consumables for those devices. [36-40] Agreeing on physical standards like the microtiter plate was an advantage for everyone in the end. [30] Using standardized tools and parts in the production process is decreasing costs and complexity due to purchasing larger quantities of the same part rather than needing multiple specific parts. The earlier standards are getting adopted and implemented in the working process the more cost efficient it will be. Manufacturers are also challenged to differentiate their product from other manufacturers in other areas than compatibility. This leads to a better competition and an overall increase in developing new technologies rather than sticking to proprietary solutions. [30, 41] For consumers on the other hand, standardization will help to easily maintain devices even if the manufacturer no longer exists. Standards need to be flexible enough to go beyond their intended purpose or otherwise it will limit their use.
Before implementing a communication standard, a holistic plan needs to be made from scientists in cooperation with IT experts. Laboratory work needs to be simplified, and the scientist needs to be supported through standard device connectivity. Users need to have a clear benefit from using a standard, otherwise digitalization efforts will not be rewarded by regular use. As long as the use of proprietary device control software is more convenient, the use of standard communication protocols is ineffective. These benefits can, for example, be achieved by automating parts of the workflow that would otherwise result in tedious manual work, like data acquisition and central data storage following FAIR data principles. Other means to support the user in the laboratory are semi-automated SOPs, where device parameters are already preset after digitally selecting the required workflow. Using a standard protocol for device communication can realize these aspects when all devices are connected by a central device control server, as demonstrated by Porr et al. [35] and Rachuri et al. [42] After successfully choosing and implementing standard communication, it is important to keep the infrastructure open to new device additions and upgrades without being a burden on laboratory workers. Two standard communication protocols are currently coexisting in bioprocess development laboratories: Standardization in Lab Automation 2 (SiLA2) and the Laboratory and Analytical Device Standard (LADS), based on Open Platform Communications Unified Architecture (OPC UA). [17, 36, 38, 40, 43, 44]
SiLA2
“SiLA's mission is to establish international standards which create open connectivity in lab automation, to enable lab digitalization.” [38] This open-source communication standard is based on a client-server infrastructure where every laboratory device is a SiLA2 server that offers a set of features. SiLA2 servers advertise themselves on the network and are discovered by SiLA2 clients like a laboratory information management system (LIMS) or the SiLA2 Manager developed by Bromig et al. [36, 40, 42] The clients recognize the offered features by the servers and can use or call them. Real-time observation of parameters is possible through the subscription to observable properties. For integration of legacy devices, Porr et al. developed a gateway module to enable SiLA communication. [45] On the software side, the gateway module has the advantage of clearly separating the tasks while still using a standard protocol. This plays a major role in scalability and maintainability. SiLA2 has been implemented in different frameworks using Java, C#, Python, or C++ for fast and easy integration of laboratory devices. [42, 45, 46]
LADS based on OPC UA
OPC UA is a standard in multiple industries for data exchange between devices independent from the device vendor. [44] Just like SiLA2, OPC UA has a client-server infrastructure. OPC clients can be connected to any OPC server that is able to collect and further process provided data from the client. The previous version, OPC Classic, was limited to the operating system Microsoft Windows; however, OPC UA is platform-independent. OPC was used as an established standard in industry even before the need for a standard communication protocol for laboratory equipment manifested itself. [44] The problem of transferring this digital communication standard from industry to bioprocess development research laboratories is the added flexibility and heterogeneity of devices that make those laboratories a lot more complex than industrial production processes when looking at the digitalization part. [43] This is why a working group from the industrial association Spectaris developed the communication protocol LADS based on OPC UA. [17, 47] “The goal of LADS is to create a manufacturer-independent, open standard for analytical and laboratory equipment.” [48] OPC UA provides a way to integrate legacy devices into the digital infrastructure by providing wrappers for existing software. [49] Implementing OPC UA is possible in different programming languages such as Java, Python, C++, or C#. [49] OPC UA is not just enabling digital connectivity inside the laboratory but also connecting research laboratories to industrial infrastructures due to OPC UA already being established in industrial environments. [17]
Digital twins
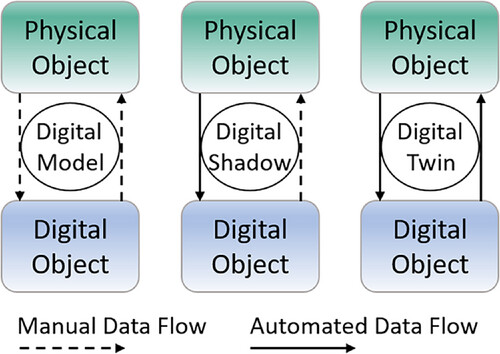
Through the development of Industry 4.0 and progressing internet of things (IoT) connectivity, the number of digital twins is increasing also in the environment of bioprocess engineering laboratories. IoT in this context describes the idea to connect physical objects like laboratory devices to a network or the internet to ensure status and information processing, as well as the ability to communicate and send and receive data. [28, 50–53] This omnipresent keyword "digital twin" is described by Fuller et al. as the “effortless integration of data between a physical and virtual machine in either direction.” [54] This concept is an invaluable advancement in bioprocess development, whereas a differentiation between "digital models," "digital shadows," and "digital twins" is important. The difference between these, as depicted in Figure 1, are the manual and automated data flows between the physical and digital object. All three concepts have a physical and digital object that are connected by data flow. Within the digital model, the data in transported manually between the physical and digital object. The digital shadow has automated data flow from the physical to the digital object, but manual data flow from the digital to the physical object. A digital twin, however, has automated data flow in both directions. Thus, only when using a digital twin, changes in the physical object will result in changes in the digital object and vice versa. [7, 54, 55]
|
Major challenges in the process of implementing digital twins in the bioprocess laboratory are data security and privacy concerns, device connectivity, trust issues regarding the use of technology, and development of a laboratory-wide digital infrastructure, including data storage space. Difficult to predict and complex processes especially in the biological laboratory complicate the development of digital twins as well. The predominant benefits on the other hand include automated data (and metadata) generation, processing, and analyzing of those as well as data traceability. All this is contributing to the aforementioned FAIR data principles. Laboratories that use digital tracking of consumables in their digital twins are able to automate their ordering system making manual inventory obsolete. Therefore, digital twins are the key technology to a new and transformed laboratory work, with the potential to automated knowledge generation. [7, 55]
Interaction with laboratory devices
Besides using standardized device communication protocols, full digitalization cannot be achieved without considering the end user. Digital solutions assisting the laboratory worker but also interacting with devices should therefore be straightforward and convenient to use. The device interactions with laboratory equipment in bioprocess development laboratories has changed over the last years, making it more efficient and intuitive with every additional solution, providing more interaction possibilities. [53]
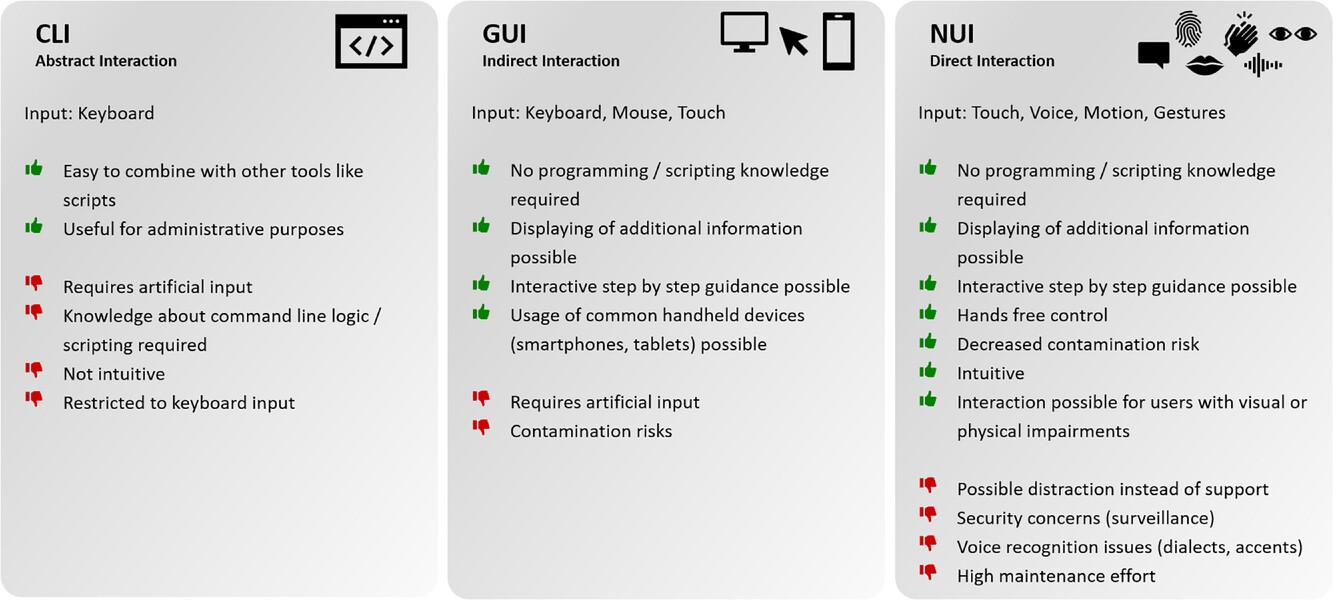
The use of laboratory devices itself is supposed to not interfere with the experiment, slow it down, or complicate it. Consequently, device operation should be as intuitive as possible. There are three different interactions levels for device control. Starting at the lowest level, there is abstract interaction, using command line interfaces (CLI), followed by the next level, the indirect interaction, using graphical user interfaces (GUI). The last and most intuitive level of device interaction is the direct interaction using natural user interfaces (NUI).
CLIs have the advantage of keyboard input from the user and are easy to integrate or combine with other tools like scripts. However, using CLIs as the interaction medium within the laboratory environment requires basic knowledge of command line logic and scripting. This skill cannot be expected from everyone working in the laboratory due to their different educational backgrounds. One approach to accelerate the transformation towards digitalized laboratories is the addition of basic IT classes (e.g., for basic scripting knowledge) to biotechnological education. However, CLIs as the only device interface are not a suitable solution for general and intuitive device interaction. Yet additional CLI device interaction may prove useful for administrative purposes or for integrating supplementary software. [56]
The next level of interaction with laboratory devices is the indirect interaction using GUIs. This has the advantage that no programming or scripting knowledge is needed for device interaction. Thus, all people in the laboratory are able to operate devices equipped with a GUI. Additionally, more information can be displayed for the user on the screen while mouse and keyboard input is enabled. A lot of devices have a vendor-specific and often proprietary control software. This often runs on a desktop PC positioned next to the device inside the laboratory. Modern devices, however, do not only have the possibility of local interaction but also offer remote interaction via network connection. Those GUIs are then deployed as touch-based network-like web interfaces, also allowing the usage of handheld devices as the end device. These handheld devices like smartphones, tablets, or touch beamers have the advantage that people are used to operating touch displays from their personal life making device interaction even more intuitive. Additionally, the use of mobile applications on smartphones and tablets is enabled. [57-59]
The use of touch-based interaction media is introducing the next level of device interaction: NUI. Besides touch-based interaction media, NUIs also include voice user interfaces (VUI) and interaction via gestures and motions. Artificial input like keyboard and mouse input becomes obsolete. This direct interaction with laboratory devices is leading to a simplified and even more intuitive way of working in the digitalized laboratory environment. Even though NUIs have a lot of advantages (Figure 2) they are not undisputed due to security concerns or voice recognition issues. [60, 61] Austerjost et al. showed in their work that VUIs can enable hands-free device control, which can be particularly advantageous in microbiology laboratories regarding contamination risks. [34] The topic of human-device interaction is described in further detail in the work of Söldner et al. [53]
|
Digitalization strategies
Abbreviations, acronyms, and initialisms
- AI: artificial intelligence
- AR: augmented reality
- CLI: command line interface
- DoE: design of experiments
- ELN: electronic laboratory notebook
- FAIR: findable, accessible, interoperable, reusable
- GUI: graphical user interface
- IoT: internet of things
- LADS: Laboratory and Analytical Device Standard
- LIMS: laboratory information management system
- ML: machine learning
- NUI: natural user interface
- OPC UA: open platform communications unified architecture
- SiLA2: Standardization in Lab Automation 2
- VR: virtual reality
- VUI: voice user interface
References
Notes
This presentation is faithful to the original, with only a few minor changes to presentation. A fair amount of grammar and punctuation was updated from the original. In some cases important information was missing from the references, and that information was added.