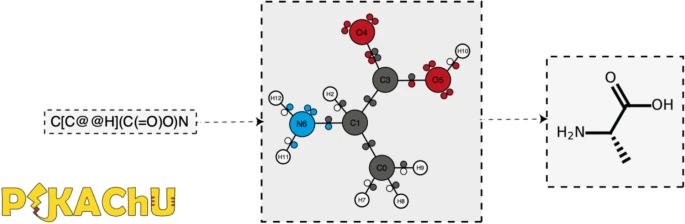
Journal:PIKAChU: A Python-based informatics kit for analyzing chemical units
| Full article title | PIKAChU: A Python-based informatics kit for analyzing chemical units |
|---|---|
| Journal | Journal of Cheminformatics |
| Author(s) | Terlouw, Barbara R.; Vromans, Sophie P.J.M.; Medema, Marnix H. |
| Author affiliation(s) | Wageningen University |
| Primary contact | barbara dot terlouw at wur dot nl |
| Year published | 2022 |
| Volume and issue | 14 |
| Page(s) | 34 |
| DOI | 10.1186/s13321-022-00616-5 |
| ISSN | 1758-2946 |
| Distribution license | Creative Commons Attribution 4.0 International |
| Website | https://jcheminf.biomedcentral.com/articles/10.1186/s13321-022-00616-5 |
| Download | https://jcheminf.biomedcentral.com/track/pdf/10.1186/s13321-022-00616-5.pdf (PDF) |
Abstract
As efforts to computationally describe and simulate the biochemical world become more commonplace, computer programs that are capable of in silico chemistry play an increasingly important role in biochemical research. While such programs exist, they are often dependency-heavy, difficult to navigate, or not written in Python, the programming language of choice for bioinformaticians.
Here, we introduce PIKAChU (Python-based Informatics Kit for Analysing CHemical Units), a cheminformatics toolbox with few dependencies implemented in Python. PIKAChU builds comprehensive molecular graphs from simplified molecular-input line-entry system (SMILES) strings, which allow for easy downstream analysis and visualization of molecules. While the molecular graphs (Graphical Abstract, below) PIKAChU generates are extensive—storing and inferring information on aromaticity, chirality, charge, hybridization, and electron orbitals—PIKAChU limits itself to applications that will be sufficient for most casual users and downstream Python-based tools and databases, such as Morgan fingerprinting, similarity scoring, substructure matching, and customizable visualization. In addition, it comes with a set of functions that assists in the easy implementation of reaction mechanisms. Its minimalistic design makes PIKAChU straightforward to use and install, in stark contrast to many existing toolkits, which are more difficult to navigate and come with a plethora of dependencies that may cause compatibility issues with downstream tools. As such, PIKAChU provides an alternative for researchers for whom basic cheminformatic processing suffices, and can be easily integrated into downstream bioinformatics and cheminformatics tools. (PIKAChU is available at https://github.com/BTheDragonMaster/pikachu.)
Keywords: cheminformatics kit, Python, structure visualization, in silico chemistry, molecular fingerprinting
Introduction
In a data-driven world where the discovery of novel natural and synthetic molecules is increasingly necessary, in silico chemical processing has become an essential part of biological and chemical research. Novel metabolites are compared or added to searchable chemical databases such as ChEBI[1], PubChem[2], NP Atlas[3], and COCONUT[4]; molecular structures are predicted from biological pathways[5][6]; and bioactivities and pharmaceutical properties are predicted from chemical structure.[7][8][9] Such analyses rely on robust cheminformatics kits that can perform basic chemical processing, such as fingerprint-based similarity searches, substructure matching, molecule visualization, and chemical features for machine learning purposes.
Typically, molecular processing by cheminformatics kits begins with the reading in of molecular data from chemical data formats, ranging from one-dimensional to three-dimensional molecular representations. One such format is the SMILES (simplified molecular-input line-entry system) format, which represents a molecule as a one-dimensional string, describing atom composition, connectivity, stereochemistry, and charge. More elaborate formats such as PDB and MOL use text files to store not just the abovementioned properties but also atom coordinates in three-dimensional space.
Depending on the application, different formats and subsequent processing are appropriate. Due to the vast number of possible chemical analyses, exhaustive cheminformatics kits have accumulated into software libraries that are so large that they can be both hard to navigate and rely on so many dependencies that they can be difficult to implement in software packages. As a result, the trade-off between time spent accessing and integrating these cheminformatics kits into a codebase and time spent on actual analyses is disproportionate for users who need to perform simple in silico analyses such as reading in SMILES, drawing a molecule, or visualizing a substructure. One popular open-source cheminformatics kit that suffers from this problem is RDKit.[10] While RDKit is an incredibly fast and powerful library that supports an immense variety of possible chemical operations, its use of both Python and C++ as programming languages, as well as the sheer number of dependencies it relies on, frequently causes compatibility issues when integrating RDKit into other programs, and disproportionately increases the number of libraries that need to be installed. Therefore, while RDKit is great for heavy-duty in silico analyses such as computing 3D conformers for a compound or constructing electron density maps, it is a bit too much for the basic operations that most researchers in bioinformatics and cheminformatics require.
A second widely-used cheminformatics kit is CDK.[11] Written in Java, it is well-suited for implementation in web applications, and it has successfully been used for molecular processing in the COCONUT database[4], the Cytoscape application chemViz2[12], and the scientific workflow platform KNIME (Konstanz Information Miner).[13] However, with Python becoming the programming language of choice for many scientists[14], especially those working in the growing field of (deep) neural networks, CDK is not always an ideal fit.
To make basic cheminformatics processing more accessible for Python programmers, we therefore introduce PIKAChU: a Python-based Informatics Kit for Analysing Chemical Units. PIKAChU is a flexible cheminformatics tool with few dependencies. It can parse molecules from SMILES, visualize chemical structures and substructures in matplotlib, perform extended connectivity fingerprinting (ECFP)[15] and Tanimoto similarity searches, and execute basic reactions with a focus on natural product chemistry. Therefore, we hope that PIKAChU can provide a convenient alternative for many Python-based bio- and cheminformatics tools and databases that only demand basic chemical processing.
Methods and implementations
Software description
PIKAChU is implemented in Python (v3.9.7). Its only dependency is the common Python package matplotlib (v3.4.3). PIKAChU can be run on Windows, MacOS, and Linux systems.
Parsing molecules from SMILES
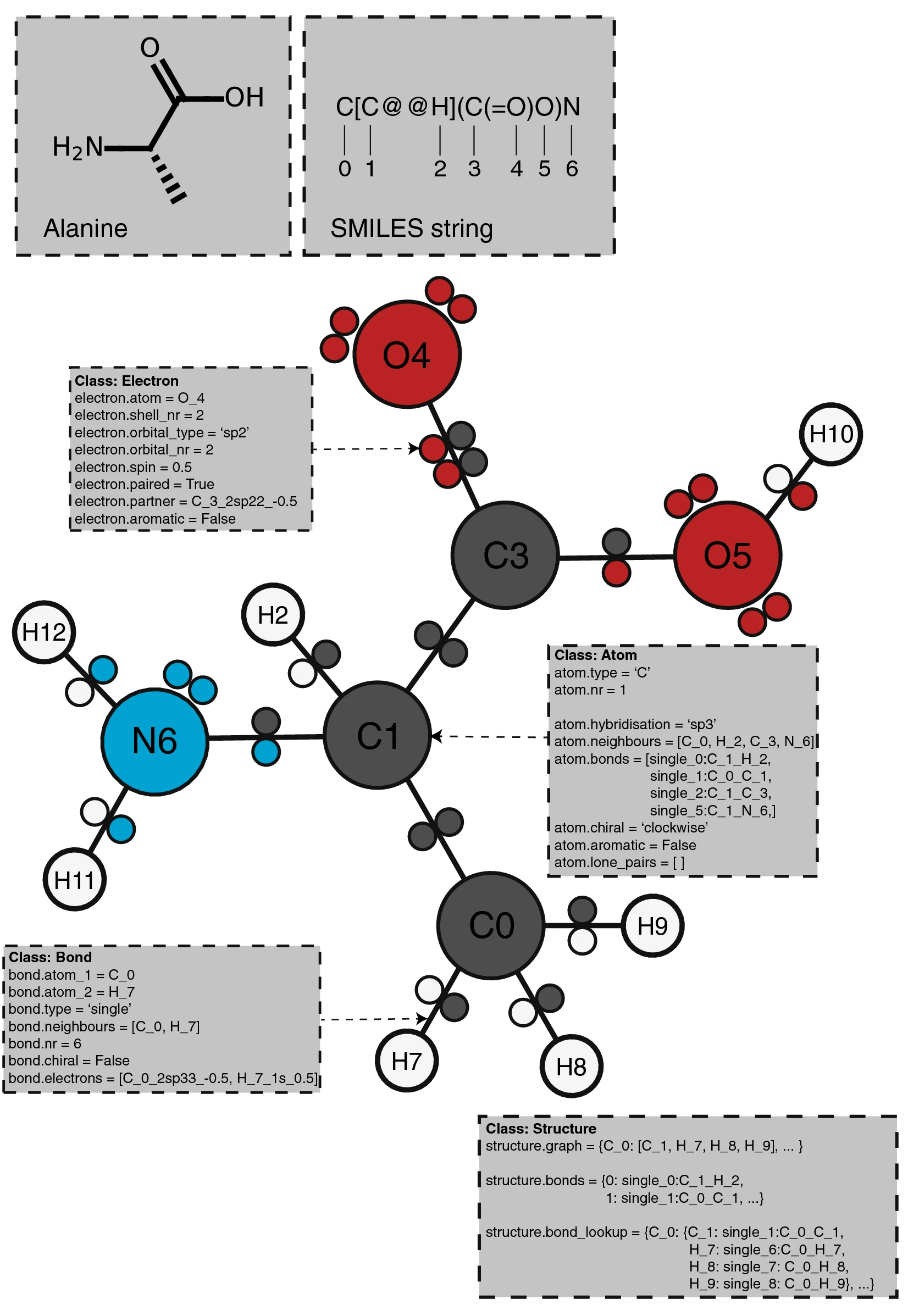
PIKAChU takes a SMILES string as input and from it builds a graph object, in which nodes represent atoms and edges represent bonds (Fig. 1). For each atom, PIKAChU initially stores information on chirality, aromaticity, charge, and connectivity. For each bond, it stores bond type (single, double, triple, quadruple, or aromatic), neighboring atoms, and cis–trans stereochemistry for double bonds. Once all atoms, bonds, and their connections have been stored, electron shells and orbitals are constructed for each non-hydrogen atom. Next, we determine the valence for each non-hydrogen atom, taking into account atom charge. For atoms of variable valence such as sulfur (2, 4 or 6) and phosphorus (3 or 5), we select a valence that is equal to or higher than the sum of non-hydrogen bonds and explicit hydrogen bonds, prioritizing smaller valences. Double, triple, quadruple, and aromatic bonds contribute proportionally to this sum. If insufficient bonding orbitals are available to achieve the desired valence, the electrons in the valence shell are excited to higher-energy orbitals, such that each orbital contains at most one electron. Implicit hydrogens are then added to the structure such that the pre-determined valences are obeyed.
|
An exception is made for nitrogen with valence 5, which is not chemically possible due to insufficient bonding orbitals but can sometimes be encountered in SMILES strings, especially in those describing compounds containing nitro groups. If such a valence 5 nitrogen is attached to at least one oxygen through a double bond, this double bond is interpreted as a single bond instead, the oxygen’s charge is set to − 1 and the nitrogen’s charge is set to 1, such that the nitrogen’s valence becomes 4 and bonding laws are obeyed.
Subsequently, electrons are allocated to the p-orbitals of π bonds in double, triple, and quadruple bonds, and atom hybridization is determined from steric number. Then, all cycles in the graph are detected using an open-source Python implementation[16] of the simple cycle detection algorithm described by Johnson in 1975.[17] PIKAChU removes all cycles smaller than three atoms and identifies the smallest set of unique smallest rings (SSSR).
Next, the SSSR is used for aromaticity detection. This is done recursively: in each round, each cycle that has not yet been added to the set of aromatic cycles is evaluated with Hückel’s 4n + 2 rule on planar rings. We chose to assess aromatic cycles rather than systems; Hückel’s rule is not always reliable for cyclic systems.[18] First, the hybridization of all atoms in the cycle is examined. All atoms must be sp2-hybridised, or sp3-hybridised with a delocalizable lone pair that can be promoted to a p-orbital. If the cycle is planar and the sum of double bonds and lone pairs is odd, the cycle is considered aromatic. Aromatic bond stretches are locally kekulized, and double bonds are subsequently counted. When a cycle is considered aromatic, bonds and atoms in the cycle are set to aromatic, and lone pairs of sp3-hybridised atoms are promoted to p-orbitals such that the new hybridization is sp2. Recursion is needed in case double bonds in cyclic systems are defined in such a way that not all sub-cycles contain the required number of bonds to obey Hückel’s rule: when adjacent bonds are updated to aromatic, they will be counted in the next round of aromaticity detection (Additional file 2: Fig. S1). When, after an iteration, the number of aromatic cycles no longer changes, all aromatic cycles have been detected. From these cycles, PIKAChU defines aromatic systems, where aromatic cycles are considered part of an aromatic system if they share a bond with at least one other aromatic cycle in the system.
Electrons involved in σ bonds and aromatic bonds are only allocated after aromaticity detection. As electrons involved in aromatic systems are not localized to specific atoms or bonds, the p-orbitals of atoms in aromatic systems are emptied and their electrons stored in an AromaticSystem object.
Finally, any unpaired electrons are dropped back to lower-energy orbitals. A structure object is returned which can be visualized, kekulized, analyzed through substructure matching and molecular fingerprinting, and altered through an assortment of built-in and custom chemical reactions.
If a SMILES string yields a structure object that is chemically incorrect due to too many or too few bonds being attached to an atom or valence shells not being filled appropriately in the case of organic atoms, PIKAChU gives a StructureError, informing the user that the parsed structure is chemically incorrect and gives a rough indication of why. Two examples of such StructureError messages are "Error parsing 'F/C(\Cl)=C(F)/Cl': Conflicting double bond stereochemistry" and "Error parsing 'CN(=O)=O': Basic bonding laws have been violated."
Visualization and kekulization
Prior to visualization, aromatic systems within a structure are kekulized so that aromatic systems can be represented by alternating single and double bonds. PIKAChU kekulizes aromatic systems using a Python implementation[19] of Edmonds’ Blossom Algorithm for maximum matching.[20] Next, atoms are positioned using PIKAChU’s drawing software. PIKAChU’s Python-based drawing algorithm was adapted and improved from SmilesDrawer[21], an open-source JavaScript library for molecular visualization. While written in different programming languages, the algorithms underlying the drawing software of PIKAChU and SmilesDrawer are largely identical. We will briefly recap this algorithm below; more detailed descriptions of the algorithm’s elements can be found in the SmilesDrawer paper.[21]
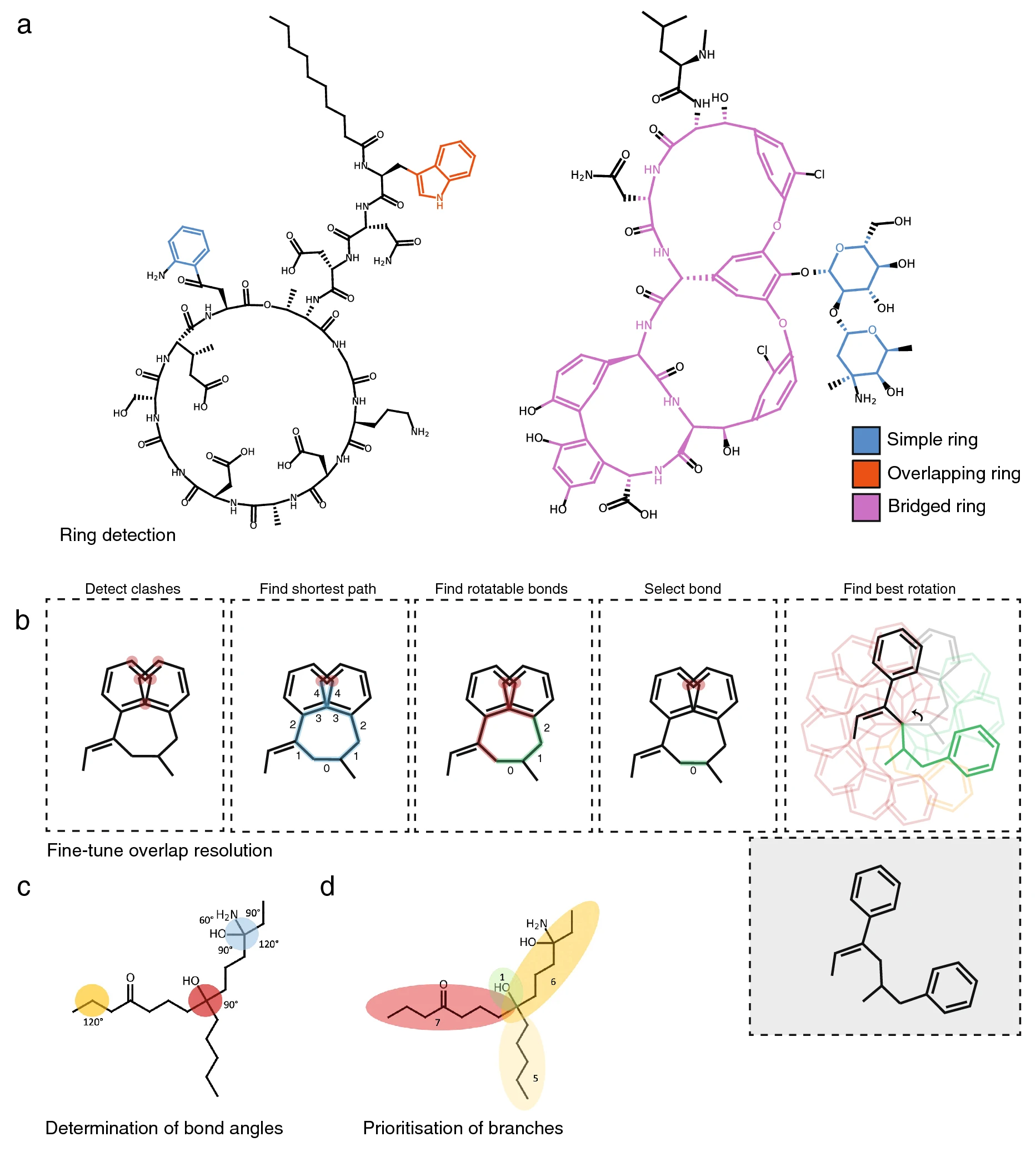
First, if indicated, PIKAChU’s drawing algorithm removes hydrogens from the graph. Next, it finds the smallest set of smallest rings in the structure graph. As SmilesDrawer’s SSSR implementation sometimes failed to detect some rings, leading to unreadable structure renderings (Additional file 2: Fig. S2), we implemented the SSSR algorithm ourselves. Next, like SmilesDrawer, PIKAChU classifies all rings into one of three groups: simple rings, overlapping rings, and bridged rings. Simple rings are standalone rings that do not have any overlapping atoms with any other rings. Overlapping rings are rings that overlap with one or more other rings, where the overlap between any two rings can comprise at most two atoms, any atom in the overlap is part of at most two rings, and no atoms in the ring overlap with bridged rings. Finally, bridged rings are rings that share more than two atoms with another ring, contain atoms that are part of three or more rings, or share atoms with another bridged ring (Fig. 2a).
|
After ring systems have been identified, atoms are placed onto a 2D coordinate system. If the molecule contains rings, positioning starts with the placement of an atom in a ring, prioritizing bridged rings over simple and overlapping rings. Then, the graph is traversed one atom at a time in depth-first fashion. If an atom is part of a ring, the entire ring or ring system get placed at once. In the case of simple and overlapping rings, ring placement can be done using simple polygon geometry. For bridged rings, atoms are positioned using the force-spring model described by Kamada and Kawai[22], where all atoms of the bridged system are initially placed in a circle, and then pulled towards their optimal positions by minimizing the difference between the desired bond length and the distance between neighboring atoms, and maximizing distances between non-neighboring atoms. Non-ring atoms are positioned a bond length away from the previous atom, where the angle with respect to the previous atom is determined by the number of neighbors the atom has (Fig. 2c), and the size of the molecular subtree behind each neighboring atom (Fig. 2d). Stereochemically restricted double bonds are always forced into the appropriate cis- or trans conformation. Unlike SmilesDrawer, which directly infers bond stereochemistry from the SMILES string, PIKAChU draws this information from bond objects stored in the molecular graph. As an improvement on SmilesDrawer, PIKAChU attempts to resolve wrongly depicted stereobonds in rings by mirroring one of the neighboring atoms into the ring. PIKAChU always selects the atom with the smallest protruding side chain for this purpose (Additional file 2: Fig. S3). When multiple consecutive stereobonds are found in a ring, PIKAChU adjusts them in order, never rotating a neighbor of the same bond twice.
Once all atoms have been assigned initial coordinates, atoms adjacent to rings are flipped outside of their ring where possible. Then, the drawing is checked for overlaps between atoms, and these overlaps are resolved by rotating branches of the molecule around single bonds. In PIKAChU, we have included an extra "fine-tuning" option that is not present in SmilesDrawer. When the fine-tuning flag is set to True, all pairs of clashing atoms are detected. Then, the shortest path is calculated between all clashing atoms. First, PIKAChU determines which bonds are rotatable: bonds are considered unrotatable when they are a chiral bond, are adjacent to a chiral bond, or are in a cycle. As rotations around bonds located equally far away from two clashing atoms likely have the greatest impact on clash resolution, PIKAChU selects the rotatable bond that is positioned as close to the center of the shortest path as possible. Next, PIKAChU takes the resulting set of bonds found for all clashes and rotates each at 30° intervals, assessing and storing the number of clashes in the drawing after each iteration. The angle for which the number of steric clashes is minimized is chosen (Fig. 2b).
Finally, some bonds adjacent to chiral centers are replaced with backward and forward wedges. They are placed such that they do not neighbor more than one chiral center where possible, they are not part of a ring, and point in the direction of the shortest branch leading from a chiral center, in that order of priority. The resulting image is subsequently written to an .svg or .png file or displayed directly in matplotlib.
Structure annotation
PIKAChU provides the option to add custom annotations to structures. Each Atom instance contains an "annotations" attribute, which points to an AtomAnnotation instance. An AtomAnnotations instance can contain as many annotations as the user requires. Annotations can be added to all atoms in a structure at once by defining the name of the attribute with a string and, optionally, providing a default value for the attribute. Subsequently, specific values can be added and retrieved for specific atoms or atom sets. A manual providing an example can be found on the PIKAChU wiki.
Substructure matching
PIKAChU detects occurrences of a substructure in a superstructure in five steps. In all steps, hydrogens are ignored. First, PIKAChU checks for each atom type in the substructure if enough atoms of these types are accounted for in the superstructure. Second, it assesses for each atom in the substructure whether an atom exists in the superstructure with the same connectivity, looking at directly neighboring bonds and atoms. Third, using the atom with the most diverse connectivity as a seed, it finds matches of the substructure in the superstructure using a depth-first search algorithm, ignoring stereochemistry. By first looking at atom type and atom connectivity, and by using atoms of diverse connectivity as seeds for substructure matching, the number of calls to the computationally expensive depth-first search function is minimized. Fourth, for each match, it determines if all chiral centers in the substructure have the same orientation as corresponding chiral centers in the superstructure. Fifth, PIKAChU checks if cis–trans orientation of double bonds in the substructure matches that of double bonds in the superstructure. Chiral center and double bond stereochemistry checks can be toggled by the user independently of one another. If chirality of bonds and atoms are considered, substructures with undefined stereochemistry will still match to parent structures with defined stereochemistry. This does not apply in reverse: if a stereocenter or stereobond is defined for a substructure, it will not match to parent structures with undefined stereochemistry.
The algorithm described is somewhat similar to the Ullmann algorithm[23], which first assesses if a candidate subgraph contains enough nodes of the correct degree prior to substructure matching and selects nodes of the most unique degree as seeds. A key difference is that PIKAChU’s substructure matching algorithm also takes into account the identity of a node’s neighbors, not just a node’s degree.
Substructures can be easily visualized through a range of functions in PIKAChU’s "general" 'library.
Fingerprinting
PIKAChU uses extended connectivity fingerprinting (ECFP)[15], an improved version of the classical Morgan fingerprinting that also takes into account cycle membership, to perform similarity searches and convert molecules to bit vectors for machine learning features. Using Python’s inbuilt hashlib library, PIKAChU initializes each atom to a 32-bit hash, derived from a tuple containing information on heavy neighbors, valence, atomic number, atomic weight, charge, hydrogen neighbors, and ring membership. Then, each atom hash is iteratively updated with hashes from its neighbors, as well as the distance from the neighbor to the atom and stereochemical information if the atom is a chiral center. The number of iterations depends on a radius which can be set to any number (default = 2 for ECFP-4 fingerprinting). (The ECFP algorithm was described in detail by Rogers and Hahn in 2010.[15]) Finally, duplicate hashes are removed, as well as different hashes representing the same substructure, yielding a set of 32-bit hashes that constitutes a molecule’s fingerprint.
Using ECFP, PIKAChU can calculate Jaccard/Tanimoto distance and/or similarity between any two molecules. Furthermore, PIKAChU can convert molecule libraries into bit vectors of varying lengths (default = 1024) and an accompanying list of substructures represented by those bit vectors that can be used in downstream machine learning algorithms.
Defining reaction targets
In order to facilitate implementation of reactions and reaction pathways, PIKAChU lets users define target bonds or atoms within substructures with a set of dedicated functions. These functions take a SMILES string representing a substructure, and either one or two integers that define an atom or a bond between two atoms, respectively. For example, the SMILES string "C(= O)NC," accompanied by the integers 0 (pointing to the first C atom) and 2 (pointing to the N atom), represents a peptide bond. The occurrences of these bonds/atoms are then detected within a superstructure through a substructure search and are returned as a list of bonds/atoms. Subsequently, the returned bonds/atoms can be used as reaction targets, for instance for bond hydrolysis or atom methylation, using functions in PIKAChU for breaking or creating bonds and adding or removing atoms. Reactions currently have to be encoded manually using a library of functions included in PIKAChU, which include functions for creating bonds, breaking bonds, adding and removing atoms, and splitting disconnected graphs into separate structures. We provided in-built condensation and hydrolysis functions, as well as a more elaborate ketoreductase function, as examples on our GitHub page.
Characterization and visualization of the polyketide ketoreduction reaction
We demonstrated the implementation of reactions using PIKAChU by characterizing and visualizing a polyketide ketoreduction reaction. We built the ketoreduction reaction by first defining a reaction target as described above, in this case a β-keto bond, and detecting its position in a polyketide chain. Next, we wrote a function that reduces the double carbonyl bond to a single bond, which identifies and removes the π-electrons in the double bond, sets the bond type to single, adjusts the hybridization of the atoms involved and finally updates the structure object through PIKAChU’s refresh functions. To finalize the reaction, two hydrogen atoms were added to the carbon and oxygen atoms of the former carbonyl bond using PIKAChU’s add_atom function. Finally, to visualize the reaction, we highlighted the atoms and bonds of the newly formed hydroxyl group in red and drew the molecule.
Detailed instructions on how to make full use of PIKAChU’s range of functionalities, as well as the script used to implement the ketoreduction reaction, can be found in the online documentation.
Validation
To assess the correctness of PIKAChU’s SMILES reading and writing software, we converted all SMILES strings from the NP Atlas database into PIKAChU Structure instances. Subsequently, we converted these structure instances back to SMILES strings. Next, we canonicalized the PIKAChU-generated SMILES and the original SMILES using RDKit (v2020.09.1.0), setting the "isomericSmiles" flag to "True" such that correct interpretation of cis–trans bond configuration and the stereochemistry of chiral centers could also be assessed. If the two canonicalized SMILES were identical, a SMILES-to-structure-to-SMILES conversion was considered correct.
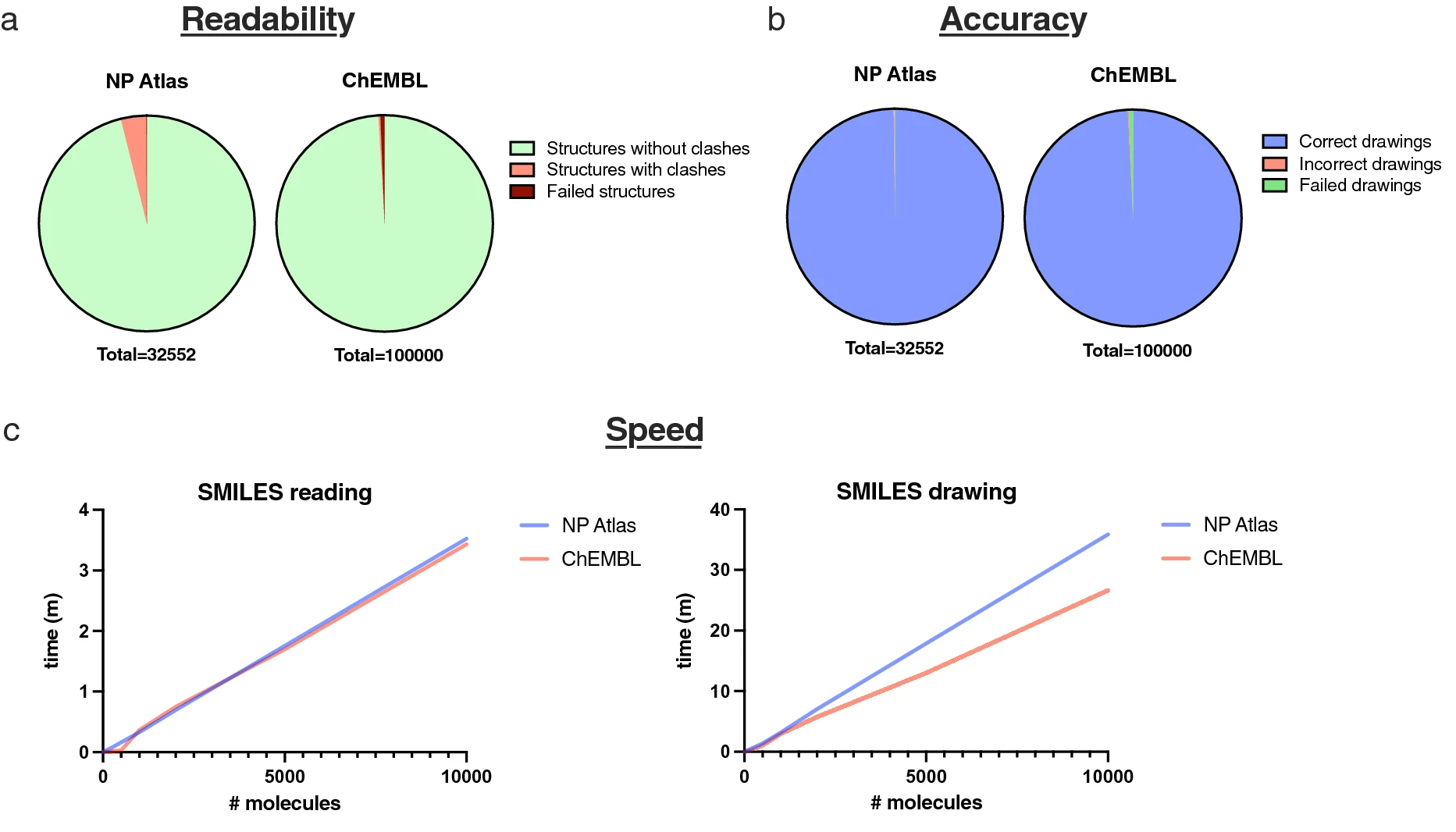
To measure PIKAChU’s drawing readability, atom coordinates were computed with PIKAChU and RDKit’s rdCoordGen module (v2020.09.1.0) for the 32,552 molecules in the NPAtlas database (v2021_08) and the 100,000 smallest molecules from the ChEMBL database (release 30). Next, all drawings were assessed for clashes. A clash was defined as two non-neighboring atoms sitting at less than half an average bond length distance from each other in Euclidean space. Total number of clashes, number of structures containing clashes, and the number of structures that gave drawing errors were recorded.
To assess PIKAChU’s drawing accuracy, we included a MOL file writer into PIKAChU, which stores PIKAChU-computed atom coordinates and connections as a MOL file. We generated such MOL files for the entire NP Atlas database and the 100,000 smallest molecules from the ChEMBL database, read the resulting MOL files with RDKit’s rdMolFiles module (v2020.09.1.0), stored the resulting molecules as SMILES strings, and using RDKit canonicalized both the original input SMILES and the SMILES produced from the PIKAChU-generated MOL files setting the "isomericSmiles" flag to "True." If the SMILES were identical, a PIKAChU-generated drawing was considered "correct."
Speed assessment
PIKAChU’s speed was assessed with Python’s "time" module. As we particularly designed PIKAChU with natural product chemistry in mind, which typically involves larger and more heavily cyclized compounds than most molecules stored in small-molecule databases, we decided to test drawing speed on two different databases: the NP Atlas database and the ChEMBL database. For each database, we randomly selected 10,000 molecules and timed drawing speed at 10, 20, 50, 100, 200, 500, 1000, 2000, 5000, and 10,000 drawn structures using Python’s "time" module.
Results and discussion
PIKAChU is a dependency-light cheminformatics kit implemented entirely in Python. With only matplotlib as dependency and an extensive readme, wiki, tutorials, and example scripts on its GitHub page, PIKAChU is easy to run and install, and suitable for integration into bioinformatics and cheminformatics pipelines. Below, we will first assess PIKAChU’s ability to correctly interpret SMILES, draw structures accurately and readably, detect and visualize substructures, and perform ECFP. We also measured PIKAChU’s SMILES reading and structure drawing speed. Next, we demonstrate how PIKAChU can be used to implement and visualize reactions. Finally, we compare PIKAChU to the state-of-the-art cheminformatics kits/chemical drawing libraries RDKit, ChemDraw, and SmilesDrawer.
SMILES reading and writing
We assessed PIKAChU’s ability to parse and generate correct SMILES syntax by comparing the SMILES it converts to SMILES generated by RDKit, a well-established cheminformatics package. As PIKAChU was created with natural product chemistry in mind, which typically involves large and heavily cyclized molecules the function of which depends heavily on stereochemistry, we performed our validation with the NP Atlas database. This database contains 32,552 manually curated natural product structures and their corresponding isomeric SMILES strings. PIKAChU failed to convert one SMILES from NP Atlas (~ 0.003%) to structure graphs, which was an erroneous SMILES describing a nitrogen atom with a valence of 5, which is impossible considering that nitrogen only has four electron orbitals available for bonding in its valence shell. This SMILES attempted to describe a nitro group, which PIKAChU tolerates even for valence 5 nitrogen types. However, this representation of a nitro group was unconventional and incorrect, with two superfluous hydrogen atoms attached to the nitrogen. This demonstrates how the detailed graph-based, object-oriented encoding of chemical structures down to the electron level in PIKACHU intrinsically ensures that all structures that are loaded are chemically valid. Of the remaining 32,551 SMILES-to-structure-graph-to-SMILES conversions, only one yielded a SMILES string that described different chemistry than the original: three carbon-13 atoms were interpreted as carbon-12 (Additional file 2: Table S1, third row). As PIKAChU does not yet support isotopic differentiation, this is not unexpected.
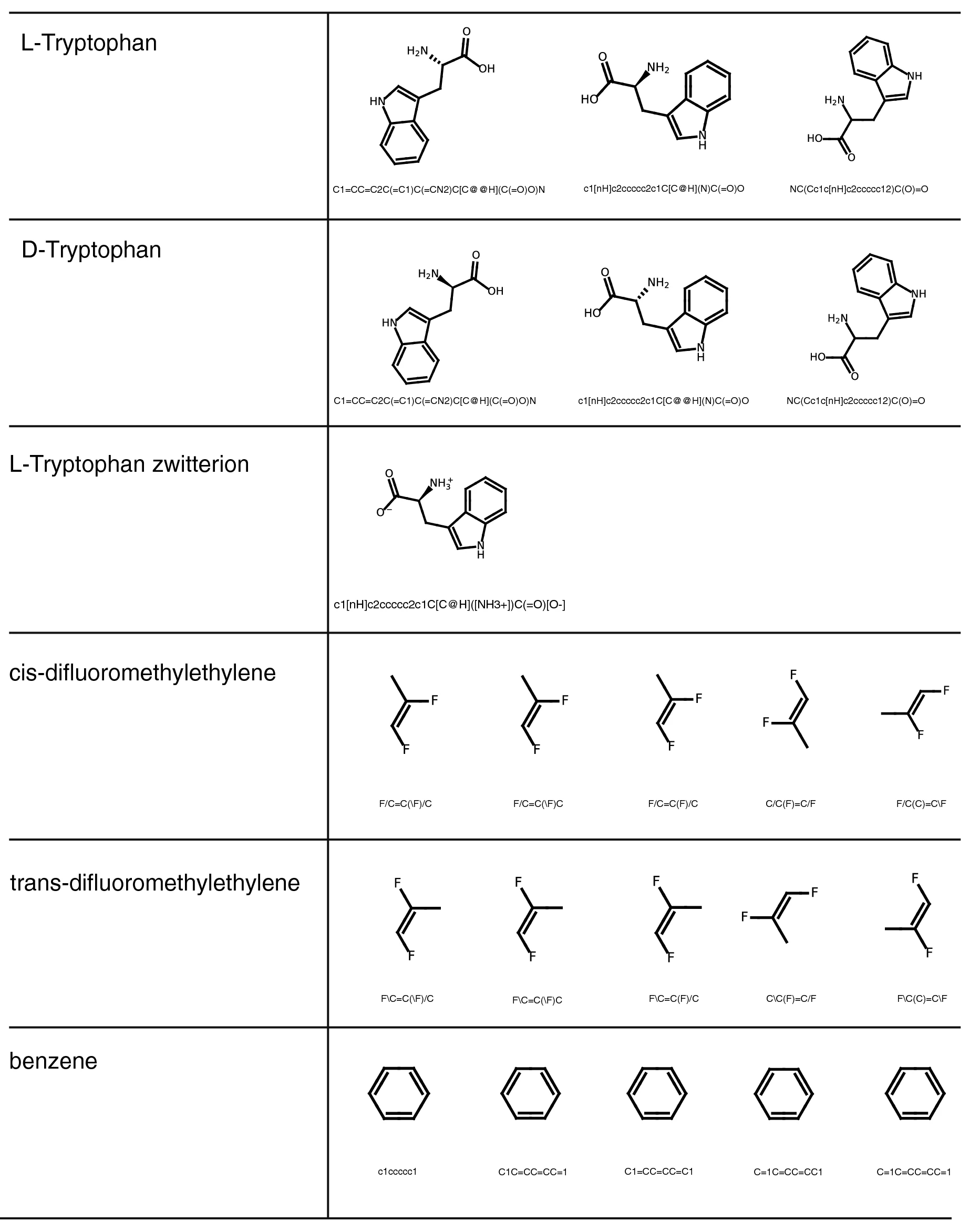
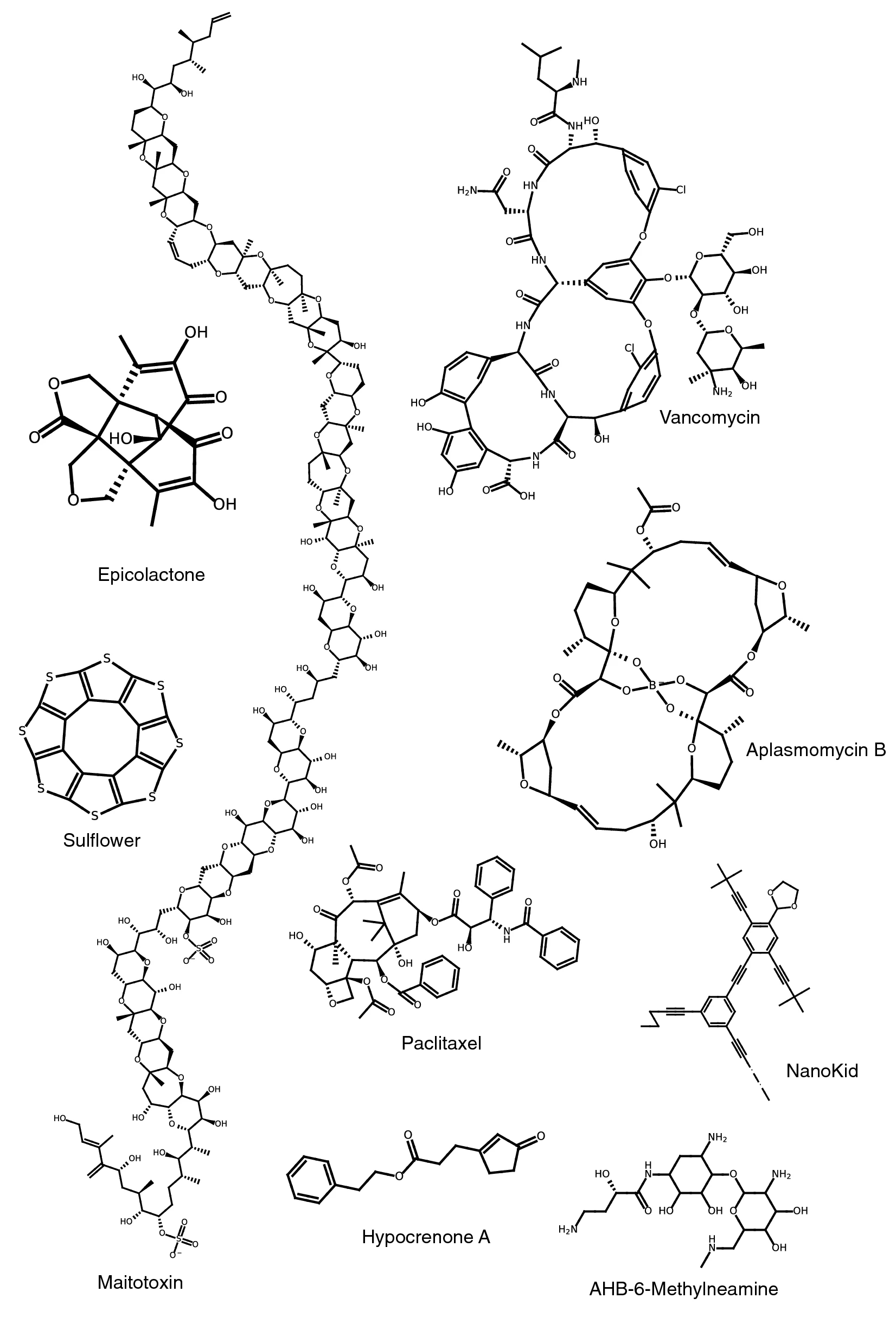
Additionally, we manually assessed the correctness of 22 SMILES-to-graph conversions by reading in and subsequently drawing the SMILES in PIKAChU. We chose the SMILES such that a variety of syntax representations and chemistries were represented, including rings, aromatic systems, charge, stereocenters, and bond stereochemistry. Some SMILES describe the same structures but use a different syntax. PIKAChU handled all SMILES correctly, accurately detecting and visualizing all aforementioned chemical properties (Fig. 3).
|
PIKAChU is not suitable for reading in molecules with a high number of recursive cycles, such as buckminsterfullerene. As PIKAChU detects all possible cycles within a molecule to determine aromaticity of cyclic systems, this step takes so long to compute that the program appears to get "stuck." However, there exist only a handful of examples of such molecules, none of which have any real practical biological or chemical relevance.
Structure visualization
Another key feature of PIKAChU is molecular visualization from SMILES. PIKAChU’s drawing software relies on similar logic to that of SmilesDrawer, a JavaScript SMILES drawing library. In our software, we added a few improvements: we fixed cis–trans stereochemistry detection (Additional file 2: Fig. S3), included an extra overlap resolution step (Fig. 2b), and implemented an improved version for finding the smallest subset of smallest rings, a key step in correctly depicting cycles. There is always a bit of debate regarding the visualization of molecular macrocycles. Many organic chemists opt for a "honeycomb" architecture, as employed by ChemDraw and CDK, to better represent the 3D architecture of a molecule, hinting at long-distance interactions that may take place within the compound (Additional file 2: Fig. S4b). However, this representation does not instantly draw the eye to sites of cyclization, a drawback for natural product biologists and bioinformaticians who are often interested in the biosynthetic steps involved in a compound’s assembly. As PIKAChU was created with natural product chemistry in mind, we chose to use a polygon representation for macrocycles, which clearly shows cyclization sites (Additional file 2: Fig. S4a).
While PIKAChU always detects and interprets aromaticity internally, it currently only supports drawing structures in a kekulized format.
The most important aspect of automated molecular visualization is accuracy: users need to be able to rely on the correctness of drawing software, especially when processing a large number of structures at once making it impossible to inspect each image independently. To this purpose, we visualized a chemically diverse set of structures from the ChEMBL and NP Atlas databases, and tested if RDKit could interpret correct chemistry from PIKAChU-generated atom coordinates. Out of the 32,552 structures in the NP Atlas database, only 40 (~ 0.12%) were drawn incorrectly. Of these, 33 were drawn wrongly due to incorrect depiction of cis–trans chemistry of double bonds adjacent to nested rings (Additional file 2: Table S1). Additionally, PIKAChU failed to convert eight structures to drawings (~ 0.02%). One of these was the same incorrectly defined SMILES describing the valence 5 nitrogen that was found previously. The remaining failures largely resulted from ChiralityErrors: errors raised by PIKAChU when it cannot correctly depict cis-trans chemistry of a double bond. With 32,504 correctly drawn structures, PIKAChU achieves a drawing accuracy of 99.85%. PIKAChU performed comparably on the 100,000 smallest molecules from the ChEMBL database (99.21% accuracy, 0.16% incorrect drawings, 0.63% unsuccessful conversions; Fig. 4a). Comprehensive lists and example depictions of SMILES leading to incorrect interpretations can be found in Additional file 2: Table S1 and Additional file 1.
|
We additionally assessed the readability of these PIKAChU-rendered drawings by automatically detecting steric clashes from PIKAChU-generated atom coordinate sets. Only 4.95% of NP Atlas SMILES renderings contained steric clashes, with ~ 1.62 clashes per clashing structure. PIKAChU was better at processing the ChEMBL database, with only ~ 0.30% of drawings containing clashing atoms. This makes sense, as NP Atlas contains a higher proportion of highly cyclized systems and large molecules, properties which make it more difficult to readably depict a molecule in a plane. As the honeycomb approach of depicting molecules solves some of these issues, we hope to implement the option to visualize molecules using either the honeycomb or the polygon strategy in the future.
In Fig. 5, we show nine examples of structures rendered by PIKAChU. Due to the drawing algorithm that PIKAChU employs for complex ring systems, 5-membered and 6-membered rings often appear distorted, as observed for vancomycin and aplasmomycin B. Additionally, PIKAChU’s overlap resolution step, while resolving a lot of steric clashes, sometimes results in carbon–carbon bonds being placed at an 180° angle which makes the structure less interpretable, as seen for PIKAChU’s depiction of the molecule nanokid.
|
Speed assessment
We assessed PIKAChU’s SMILES reading and structure drawing speed by drawing 10,000 random molecules from the NP Atlas database and the ChEMBL database (Fig. 4c). With an average reading speed of ~ 2,874 SMILES per minute and an average drawing speed of ~ 279 molecules per minute for the NP Atlas database and ~ 375 molecules per minute for the ChEMBL database on a single laptop core, it is clear that PIKAChU spends the bulk of its time rendering an image on atom positioning, not on SMILES reading. The discrepancy between the two databases can be explained by the nature of the molecules contained within them: typically, natural products are larger and more cyclized than the average small molecule. This makes PIKAChU’s drawing speed one order of magnitude slower than RDKit’s (Additional file 2: Table S2), which is expected considering that PIKAChU is a pure Python package while RDKit generates drawings with pre-compiled C++ code. Also, PIKAChU’s fine-tuning step is computationally expensive, likely leading to an increase in computational time. Still, PIKAChU is fast enough for integration into pure-Python bioinformatics and cheminformatics pipelines.
Substructure detection
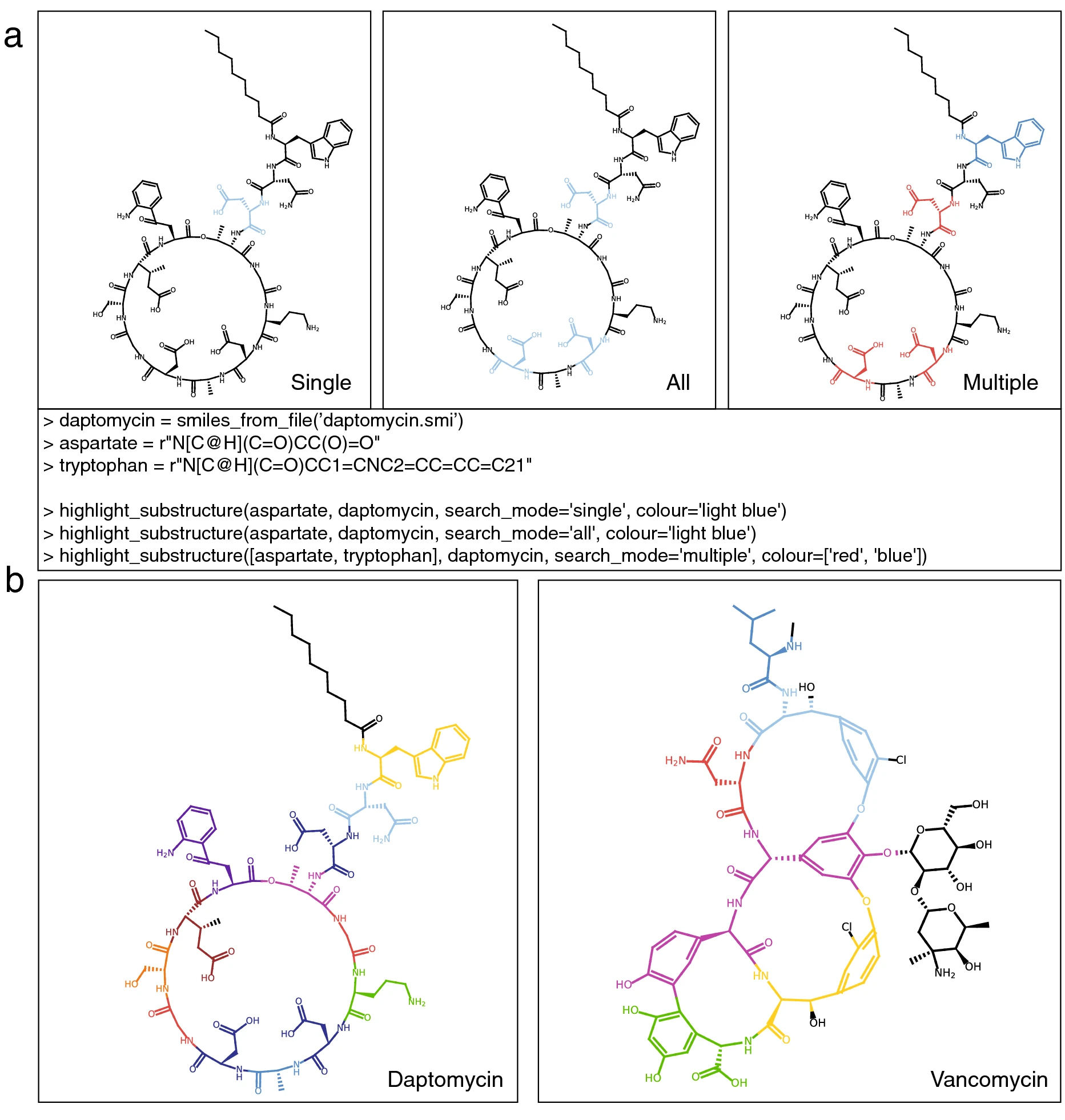
A dedicated set of functions ensures that performing substructure searches using PIKAChU is straightforward. With a single line of code, users can visualize a single occurrence of a substructure, all occurrences of a substructure, or all occurrences of a range of substructures in a chemical compound (Fig. 6a). Substructure searches are fast due to several pre-processing steps, ensuring that the expensive graph matching algorithm is only executed when a match is likely. Stereochemistry matching, activated by default, can be toggled on and off.
With PIKAChU’s substructure matching algorithm, we visualized the amino acid composition of the cyclic peptides daptomycin and vancomycin using only a single line of code for each (Fig. 6b). Colors are fully and easily customizable and can be provided as hex codes or as color names.
|
ECFP fingerprinting
To quickly determine the approximate similarity between two molecules, PIKAChU employs ECFP.[15] PIKAChU hashes each molecule into a set of unique identifiers, each of which represents a substructure. Collectively, these identifiers make up a molecule’s fingerprint. Then, PIKAChU calculates the Jaccard/Tanimoto similarity between two molecules by comparing their fingerprints, giving a measure of molecular similarity and/or distance.
Here, we showcase PIKAChU’s ECFP by calculating and subsequently constructing a tSNE plot of the molecular distances between 36 calcium-dependent lipopeptides. Lipopeptides of the same family grouped together (Additional file 2: Fig. S5), confirming that PIKAChU’s ECFP yields reliable measures of chemical similarity.
Additionally, PIKAChU’s ECFP makes it possible to generate bit vectors from molecule sets, where each element in the vector represents the presence/absence of a specific substructure. These can subsequently be used as interpretable molecular features for machine learning.
Building in silico reactions using PIKAChU
PIKAChU provides a platform for the creation and visualization of reaction mechanisms by providing a range of reaction functions that can be used to make or break molecular bonds, add or remove atoms, and alter the chirality of stereocenters. In addition to these built-in reaction building blocks, PIKAChU allows users to easily define more complex reactions through the manipulation of atom and bond object attributes. Additionally, PIKAChU supports fully customizable structure annotation, which is useful for keeping track of reaction steps, reaction targets, or atom origin. As a proof of principle, we used PIKAChU to define and visualize a polyketide ketoreduction reaction, catalyzed by a ketoreductase polyketide synthase domain during polyketide synthesis, employing both built-in and custom reaction functions (Additional file 2: Fig. S6). This example, as well as a comprehensive guide containing instructions on how to build reaction mechanisms using PIKAChU, can be found in the online documentation.
While creating reaction pathways with PIKAChU enforces chemically correct conversions by checking at each step if a structure is chemically correct, it is more laborious than similar functionalities in other cheminformatics kits such as RDKit, which use reaction SMILES and atom mapping to perform chemical reactions. We intend to implement reaction SMILES and atom mapping into PIKAChU in the future.
PIKAChU compared to state-of-the-art chemical drawing software
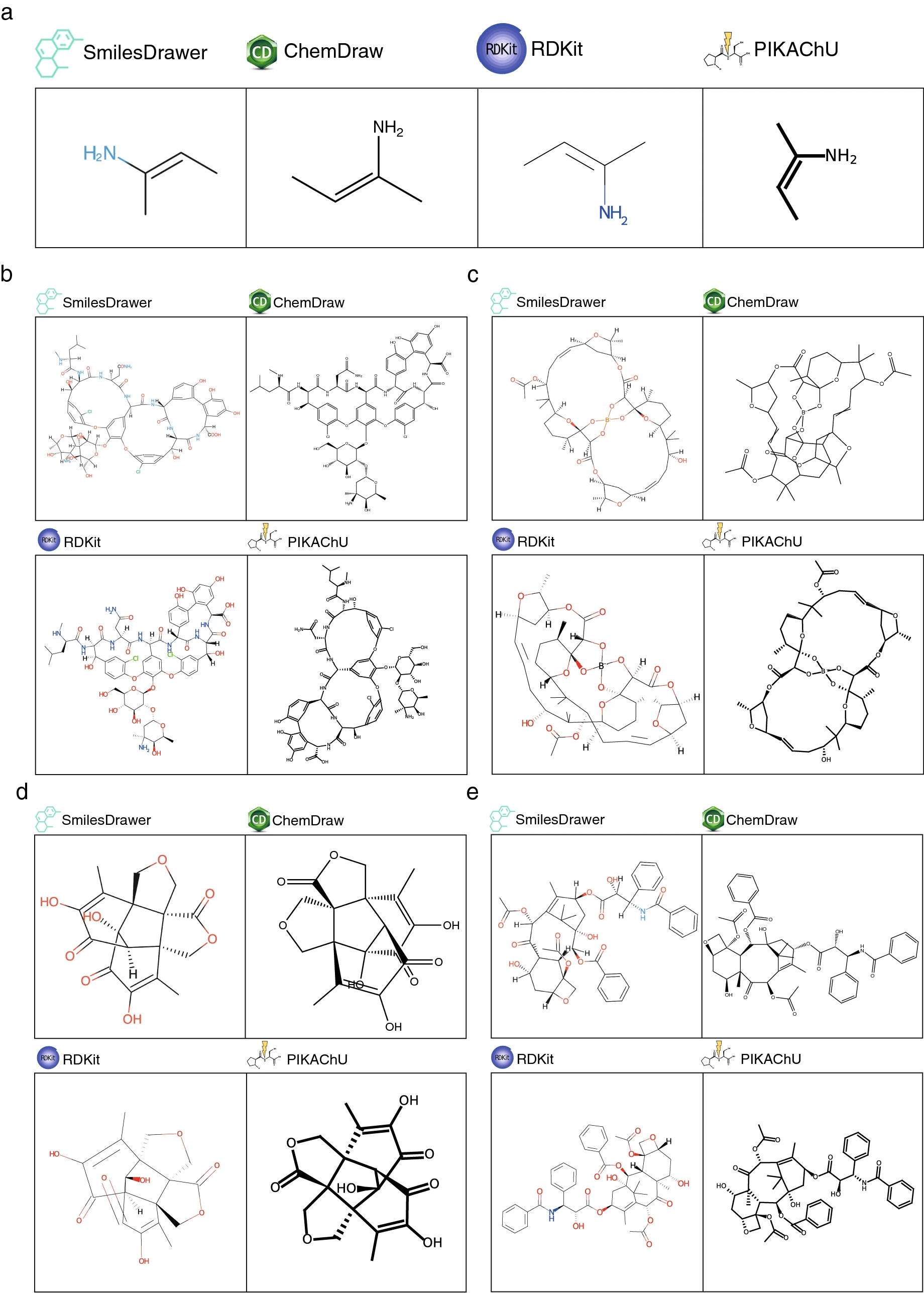
Finally, we assessed how PIKAChU performs compared to existing chemical drawing software. To this purpose, we visualized various structures in PIKAChU (v1.0.5), RDKit (v2020.09.1.0), ChemDraw (v20.1.0.112), and SmilesDrawer (v1,2.0), and manually assessed drawing quality and correctness (Fig. 7). Only SmilesDrawer occasionally produced an incorrect structure, confusing cis–trans stereochemistry when stereochemistry is defined in or after a branch (Fig. 7a). For heavily cyclized molecules (Fig. 7b–e), we see a difference between the "honeycomb" (RDKit and ChemDraw) and the "polygon" (PIKAChU and SmilesDrawer) approaches of cycle positioning. The honeycomb approach ensures minimal distortion of microcycles, even when they are part of larger systems; as such, RDKit and ChemDraw render molecules such as vancomycin with fewer distortions than SmilesDrawer and PIKAChU (Fig. 7b). However, when the honeycomb approach does not work because of steric constraints, forcing microcycles into regular polygons can distort the macrocyclic structure to the extent that the drawing becomes unreadable. This is the case for aplasmomycin B (Fig. 7c), which is drawn with fewer bond overlaps by SmilesDrawer and PIKAChU. In structures where microcycles and macrocycles are separate, there is little difference in structure rendering between the two approaches (Fig. 7d, e). It must be said that while readable, these depictions are still far from optimal, and chemists could produce better diagrams by manually tweaking the drawings of these molecules in ChemDraw. This demonstrates that even with state-of-the-art software, two-dimensional automatic visualization remains a challenge, particularly for constrained ring systems.
|
PIKAChU has a slight advantage over RDKit in drawing molecules of varying sizes, automatically adjusting the canvas size based on the size of the molecule to be drawn. This also means that PIKAChU’s font size, bond length, and bond thickness maintain a constant ratio across different drawings, which is not the case for RDKit (Fig. 7d, e). While it is possible to manually adjust canvas size in RDKit, some extra coding steps are required to achieve this.
PIKAChU’s visual output is far more customizable than that of SmilesDrawer, allowing for molecule rotation, drawing multiple molecules on a single canvas, and custom coloring of each individual bond and atom, supporting hex-codes as well as a range of descriptive strings.
While ChemDraw accommodates high-quality and highly customizable visualization, it is not open-source. This makes PIKAChU more suitable for integration into the automated open-source pipelines required by many projects.
Conclusions
We developed PIKAChU, a dependency-light cheminformatics library implemented entirely in Python. Having extensively validated our software, we conclude that, while RDKit heavily outperforms PIKAChU in terms of speed, PIKAChU performs sufficiently fast and reliably to be suitable for cheminformatics and bioinformatics pipelines. Backed by extensive online documentation, easy and straightforward installation, and state-of-the-art automated visualization software, we hope that PIKAChU can provide a convenient alternative for chem- and bioinformaticians programming in Python.
Abbreviations, acronyms, and initialisms
ECFP: extended connectivity fingerprinting
PIKAChU: Python-based Informatics Kit for Analysing Chemical Units
SMILES: simplified molecular-input line-entry system
Supplementary information
- Additional file 1 (.xlsx): True failed SMILES. SMILES failed because of incorrect chemistry.
- Additional file 2: Figure S1 (.docx): PIKAChU’s recursive aromaticity detection. Aromatic cycles are individually and recursively detected and later joined into cyclic systems. Figure S2. Faulty ring detection by SmilesDrawer leads to unreadable structure renderings. SmilesDrawer’s SSSR implementation fails to detect one of the macrocycles, leading to the unreadable structure shown on the right. PIKAChU’s SSSR implementation (left) does recognize this ring and therefore renders the structure correctly. Figure S3. PIKAChU resolves incorrectly drawn chiral bonds in rings. PIKAChU (left) correctly depicts cis-trans chemistry of stereobonds in rings that SmilesDrawer (right) cannot visualize. Figure S4. Two approaches for visualizing macrocycles. (a) Daptomycin visualized using the 'polygon' approach in PIKAChU. (b) Daptomycin visualised using the "honeycomb" approach in ChemDraw. Figure S5. tSNE plot of 36 calcium-dependent lipopeptides drawn in a 2D plane based on the Tanimoto distances between their structures, as computed by PIKAChU. The script and structures used to draw this figure can be found in the "example_scripts" and "example_structures" folders in GitHub, respectively. Figure S6. Visualization of the polyketide ketoreduction reaction, built and visualized by PIKAChU. The reduced group is highlighted in red. The script performing this reaction can be found on GitHub. Supplementary Table S1. Examples of SMILES that PIKAChU does not draw correctly, compared to ChemDraw drawings drawn from the same SMILES. Supplementary Table S2. Drawing times of molecules rendered by RDKit and PIKAChU. Time is indicated in minutes.
Acknowledgements
Daniel Probst for providing an in-depth explanation of the SmilesDrawer software; Rutger Bosch for reporting software bugs; Zach Reitz, Joris Louwen, Lotte Pronk, David Meijer, Hannah Augustijn, Kumar Singh, Huali Xie, Jiayi Jing, Mohammad Alanjary, Chrats Melkonian, Justin van der Hooft, and Catarina Sales E. Santos Loureiro for testing the PIKAChU software.
Author contributions
BRT: Development and testing of the PIKAChU software, wrote the initial version of the manuscript and further edited it based on the suggestions of the other authors. SPJMV: Significant contributions to writing the manuscript, extensive testing and debugging of the PIKAChU software, writing and executing example scripts. MHM.: Significant contributions to writing the manuscript, suggestions for software features, research supervision. All the authors read and approved the final manuscript.
Funding
This work was supported by the Novel Antibacterial Compounds and Therapies Antagonising Resistance program (NACTAR) from the Dutch Research Council (NWO; project number 16440).
Availability of data and materials
The PIKAChU software is made available under an open-source (MIT) license and can be found at https://github.com/BTheDragonMaster/pikachu. A wiki can be found at https://github.com/BTheDragonMaster/pikachu/wiki. Scripts used for the results section of this paper are made available at https://github.com/BTheDragonMaster/pikachu/tree/main/example_scripts. The NPAtlas database used in our analyses can be downloaded at https://www.npatlas.org/download.
Competing interests
M.H.M. is a member of the Scientific Advisory Board of Hexagon Bio and co-founder of Design Pharmaceuticals.
References
- ↑ Hastings, Janna; Owen, Gareth; Dekker, Adriano; Ennis, Marcus; Kale, Namrata; Muthukrishnan, Venkatesh; Turner, Steve; Swainston, Neil et al. (4 January 2016). "ChEBI in 2016: Improved services and an expanding collection of metabolites" (in en). Nucleic Acids Research 44 (D1): D1214–D1219. doi:10.1093/nar/gkv1031. ISSN 0305-1048. PMC PMC4702775. PMID 26467479. https://academic.oup.com/nar/article-lookup/doi/10.1093/nar/gkv1031.
- ↑ Kim, Sunghwan; Chen, Jie; Cheng, Tiejun; Gindulyte, Asta; He, Jia; He, Siqian; Li, Qingliang; Shoemaker, Benjamin A et al. (8 January 2021). "PubChem in 2021: new data content and improved web interfaces" (in en). Nucleic Acids Research 49 (D1): D1388–D1395. doi:10.1093/nar/gkaa971. ISSN 0305-1048. PMC PMC7778930. PMID 33151290. https://academic.oup.com/nar/article/49/D1/D1388/5957164.
- ↑ van Santen, Jeffrey A.; Jacob, Grégoire; Singh, Amrit Leen; Aniebok, Victor; Balunas, Marcy J.; Bunsko, Derek; Neto, Fausto Carnevale; Castaño-Espriu, Laia et al. (27 November 2019). "The Natural Products Atlas: An Open Access Knowledge Base for Microbial Natural Products Discovery" (in en). ACS Central Science 5 (11): 1824–1833. doi:10.1021/acscentsci.9b00806. ISSN 2374-7943. PMC PMC6891855. PMID 31807684. https://pubs.acs.org/doi/10.1021/acscentsci.9b00806.
- ↑ 4.0 4.1 Sorokina, Maria; Merseburger, Peter; Rajan, Kohulan; Yirik, Mehmet Aziz; Steinbeck, Christoph (1 December 2021). "COCONUT online: Collection of Open Natural Products database" (in en). Journal of Cheminformatics 13 (1): 2. doi:10.1186/s13321-020-00478-9. ISSN 1758-2946. PMC PMC7798278. PMID 33423696. https://jcheminf.biomedcentral.com/articles/10.1186/s13321-020-00478-9.
- ↑ Blin, Kai; Shaw, Simon; Kloosterman, Alexander M; Charlop-Powers, Zach; van Wezel, Gilles P; Medema, Marnix H; Weber, Tilmann (2 July 2021). "antiSMASH 6.0: improving cluster detection and comparison capabilities" (in en). Nucleic Acids Research 49 (W1): W29–W35. doi:10.1093/nar/gkab335. ISSN 0305-1048. PMC PMC8262755. PMID 33978755. https://academic.oup.com/nar/article/49/W1/W29/6274535.
- ↑ Skinnider, Michael A.; Johnston, Chad W.; Gunabalasingam, Mathusan; Merwin, Nishanth J.; Kieliszek, Agata M.; MacLellan, Robyn J.; Li, Haoxin; Ranieri, Michael R. M. et al. (1 December 2020). "Comprehensive prediction of secondary metabolite structure and biological activity from microbial genome sequences" (in en). Nature Communications 11 (1): 6058. doi:10.1038/s41467-020-19986-1. ISSN 2041-1723. PMC PMC7699628. PMID 33247171. http://www.nature.com/articles/s41467-020-19986-1.
- ↑ Alvarsson, Jonathan; Lampa, Samuel; Schaal, Wesley; Andersson, Claes; Wikberg, Jarl E. S.; Spjuth, Ola (1 December 2016). "Large-scale ligand-based predictive modelling using support vector machines" (in en). Journal of Cheminformatics 8 (1): 39. doi:10.1186/s13321-016-0151-5. ISSN 1758-2946. PMC PMC4980776. PMID 27516811. https://jcheminf.biomedcentral.com/articles/10.1186/s13321-016-0151-5.
- ↑ Stokes, Jonathan M.; Yang, Kevin; Swanson, Kyle; Jin, Wengong; Cubillos-Ruiz, Andres; Donghia, Nina M.; MacNair, Craig R.; French, Shawn et al. (1 February 2020). "A Deep Learning Approach to Antibiotic Discovery" (in en). Cell 180 (4): 688–702.e13. doi:10.1016/j.cell.2020.01.021. PMC PMC8349178. PMID 32084340. https://linkinghub.elsevier.com/retrieve/pii/S0092867420301021.
- ↑ Volkamer, A.; Kuhn, D.; Rippmann, F.; Rarey, M. (1 August 2012). "DoGSiteScorer: a web server for automatic binding site prediction, analysis and druggability assessment" (in en). Bioinformatics 28 (15): 2074–2075. doi:10.1093/bioinformatics/bts310. ISSN 1367-4803. https://academic.oup.com/bioinformatics/article-lookup/doi/10.1093/bioinformatics/bts310.
- ↑ "RDKit: Open-Source Cheminformatics Software". GitHub. http://www.rdkit.org/. Retrieved 07 November 2021.
- ↑ Willighagen, Egon L.; Mayfield, John W.; Alvarsson, Jonathan; Berg, Arvid; Carlsson, Lars; Jeliazkova, Nina; Kuhn, Stefan; Pluskal, Tomáš et al. (1 December 2017). "The Chemistry Development Kit (CDK) v2.0: atom typing, depiction, molecular formulas, and substructure searching" (in en). Journal of Cheminformatics 9 (1): 33. doi:10.1186/s13321-017-0220-4. ISSN 1758-2946. PMC PMC5461230. PMID 29086040. https://jcheminf.biomedcentral.com/articles/10.1186/s13321-017-0220-4.
- ↑ "chemViz2: Cheminformatics App for Cytoscape". RBVI Cytoscape Apps. Regents of the University of California. 23 February 2015. http://www.rbvi.ucsf.edu/cytoscape/chemViz2/.
- ↑ Beisken, Stephan; Meinl, Thorsten; Wiswedel, Bernd; de Figueiredo, Luis F; Berthold, Michael; Steinbeck, Christoph (1 December 2013). "KNIME-CDK: Workflow-driven cheminformatics" (in en). BMC Bioinformatics 14 (1): 257. doi:10.1186/1471-2105-14-257. ISSN 1471-2105. PMC PMC3765822. PMID 24103053. https://bmcbioinformatics.biomedcentral.com/articles/10.1186/1471-2105-14-257.
- ↑ Cass, S. (24 August 2021). "Top Programming Languages 2021". IEEE Spectrum. https://spectrum.ieee.org/top-programming-languages-2021. Retrieved 07 November 2021.
- ↑ 15.0 15.1 15.2 15.3 Rogers, David; Hahn, Mathew (24 May 2010). "Extended-connectivity fingerprints". Journal of Chemical Information and Modeling 50 (5): 742–754. doi:10.1021/ci100050t. ISSN 1549-960X. PMID 20426451. https://pubmed.ncbi.nlm.nih.gov/20426451.
- ↑ "qpwo / python-simple-cycles". GitHub. https://github.com/qpwo/python-simple-cycles. Retrieved 21 August 2021.
- ↑ Johnson, Donald B. (1 March 1975). "Finding All the Elementary Circuits of a Directed Graph" (in en). SIAM Journal on Computing 4 (1): 77–84. doi:10.1137/0204007. ISSN 0097-5397. http://epubs.siam.org/doi/10.1137/0204007.
- ↑ Hückel, Erich (1 March 1931). "Quantentheoretische Beiträge zum Benzolproblem: I. Die Elektronenkonfiguration des Benzols und verwandter Verbindungen" (in de). Zeitschrift für Physik 70 (3-4): 204–286. doi:10.1007/BF01339530. ISSN 1434-6001. http://link.springer.com/10.1007/BF01339530.
- ↑ "yorkyer / edmonds-blossom". GitHub. https://github.com/yorkyer/edmonds-blossom. Retrieved 24 August 2021.
- ↑ Edmonds, Jack (1965). "Paths, Trees, and Flowers" (in en). Canadian Journal of Mathematics 17: 449–467. doi:10.4153/CJM-1965-045-4. ISSN 0008-414X. https://www.cambridge.org/core/product/identifier/S0008414X00039419/type/journal_article.
- ↑ 21.0 21.1 Probst, Daniel; Reymond, Jean-Louis (22 January 2018). "SmilesDrawer: Parsing and Drawing SMILES-Encoded Molecular Structures Using Client-Side JavaScript" (in en). Journal of Chemical Information and Modeling 58 (1): 1–7. doi:10.1021/acs.jcim.7b00425. ISSN 1549-9596. https://pubs.acs.org/doi/10.1021/acs.jcim.7b00425.
- ↑ Kamada, Tomihisa; Kawai, Satoru (1 April 1989). "An algorithm for drawing general undirected graphs" (in en). Information Processing Letters 31 (1): 7–15. doi:10.1016/0020-0190(89)90102-6. https://linkinghub.elsevier.com/retrieve/pii/0020019089901026.
- ↑ Ullmann, J. R. (1 January 1976). "An Algorithm for Subgraph Isomorphism" (in en). Journal of the ACM 23 (1): 31–42. doi:10.1145/321921.321925. ISSN 0004-5411. https://dl.acm.org/doi/10.1145/321921.321925.
Notes
This presentation is faithful to the original, with only a few minor changes to presentation. In some cases important information was missing from the references, and that information was added. The original article lists references alphabetically, but this version — by design — lists them in order of appearance. The original URL for the Cass article concerning popular programming languages was found to be dead; a working URL was found and replaced for this version.