Journal:Academic-industry partnership advancing cannabis science: The Complementary Care Practice-Based Research Network
| Full article title | Academic-industry partnership advancing cannabis science: The Complementary Care Practice-Based Research Network |
|---|---|
| Journal | Complementary Therapies in Medicine |
| Author(s) | Ennis, Nicole; Vance, Cameron; Bradbury, Russell |
| Author affiliation(s) | Florida State University, Medical Marijuana Treatment Clinics of Florida |
| Primary contact | Email: nennis at fsu dot edu |
| Year published | 2022 |
| Volume and issue | 66 |
| Article # | 102821 |
| DOI | 10.1016/j.ctim.2022.102821 |
| ISSN | 1873-6963 |
| Distribution license | Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International |
| Website | https://www.sciencedirect.com/science/article/pii/S0965229922000231 |
| Download | https://www.sciencedirect.com/science/article/pii/S0965229922000231/pdfft (PDF) |
Abstract
Objectives: Data collected during routine care holds the potential to support hypothesis generation, study feasibility, and provide insight regarding how to address problems under real world conditions. Currently there are no practice-based research networks in Florida that focus on complementary care in general or medical marijuana specifically. Through an academic-industry partnership, we sought to develop a practice-based research network focused on Cannabis science and create a de-identified database for analyses that support hypothesis generation, study feasibility estimation, and a network that also facilitates recruitment into future research studies.
Design: The Complementary Care Practice-Based Research Network (CC-PBRN) is a centralized repository, which contains electronic health records (EHRs) from a private medical Cannabis health system in the state of Florida.
Results: This paper provides cross-sectional descriptive analyses of the CC-PBRN’s 43,802 currently active patients. There are 24 clinics in the network, with nine in North Florida, 11 in Central Florida, and four in South Florida.
Conclusion: This network serves as a statewide resource for patient-reported outcomes (PRO) research in medical marijuana. The network currently has numerous outpatient practices with thousands of patients that are a potential source of research participants for both observational studies as well as traditional clinical trials. The outpatient clinical practices can also serve as units of analysis for pragmatic trials comparing different care protocols and organizational structure.
Keywords: complementary care, Cannabis science, practice-based research network
Introduction
Across all sectors of industry, vast amounts of data regarding health and health outcomes are collected on a routine basis.[1] Data collected during routine care holds the potential to support hypothesis generation, study feasibility, and provide insight regarding how to address problems under real-world conditions. Therefore, successful academic-industry partnerships, which put unused data from industry to work, have the potential to advance science and have significant public health impact.[1][2][3] However, there are numerous limitations that prevent the use of data collected by industry for the greater good of scientific innovation.[4] Traditional models of data management require academics to collect their own data, and industry uses the data they collect to advance their business models. Because academic researchers require academic freedom to report the data as they see fit whether or not it aligns with the business model, the traditional model of collaboration with academics in the era of big data often fails.[1]
Currently there are no practice-based research networks in Florida that focus on complementary care in general or medical marijuana specifically. Practice-based research networks started in the 1970s to provide data on primary care health concerns.[5] Today, these networks have developed and serve multiple functions, such as focusing on patient-oriented outcomes, improving delivery and quality of care, practice-based transformation model, community health improvement, and patient engagement.[6][7][8][9]
Through an academic-industry partnership, we sought to develop a practice-based research network focused on Cannabis science to create a de-identified database for analyses that support hypothesis generation, study feasibility estimation, and a network that also facilitates recruitment into future research studies. The aims of this paper are to: (1) describe the process utilized in the development of the Complementary Care Practice-Based Research Network (CC-PBRN), (2) present baseline descriptive characteristics of the practice-based research network, and (3) discuss the challenges and lessons learned in initiating an academic-industry partnership.
Developing the practice-based research network
The CC-PBRN is an academic-industry partnership between Florida State University’s intervention Research Advancing Care Equity Lab (FSU-iRACE) and Medical Marijuana Treatment Clinics of Florida (MMTC) that serves the advancement of medical Cannabis science through patient-reported outcomes. In order to ensure a successful partnership, FSU-iRACE and MMTC first met to identify the challenges a partnership could create and to develop solutions that worked with the culture of both organizations. The three Cs of collaboration, coordination, and cooperation, a traditional method of partnership building in inter-organizational relationships[10], was used as the framework to develop a resilient academic-industry partnership. A combination of in-person and virtual platform meetings allowed for more frequent meetings and supported the development of the partnership, which started July 2020. The first several meetings consisted of collaboration building, which involved the teams from each organization getting to know the organizational structure of their potential partner in a more detail-oriented manner[10][11][12], specifically concerning how each organization worked independently and the purpose collaboration would serve for each organization. By the third meeting, a "strengths, weaknesses, opportunities, and threats" (SWOT) analysis was completed in order to understand the resources that were available within each organization.[13][14] The SWOT then served as the foundation for why the organizations should collaborate and enabled operationalization of a mission for the collaborative partnership.
The next major step was coordination, which required a review of operations on both sides.[15][16] It was integral for FSU-iRACE researchers to understand what data was collected, how it was collected, how it is stored, and how it is governed. It was equally as important for MMTC to understand what data management and computing resources, research expertize, data analysis capabilities, and grant writing skills FSU-iRACE researchers currently had available to devote to the project. Once the mechanics of operations were understood, FSU-iRACE researchers drafted a concrete plan with actionable items that were assigned to each organization. That plan was sent to MMTC for review and approval. After MMTC approved the plan, negotiations for how to implement that plan moved beyond the FSU-iRACE researchers and MMTC business owners. A formal data use agreement and a business associate agreement were identified as the primary and appropriate governing structures for this partnership. At this stage of cooperation, both FSU-iRACE and MMTC referred the draft collaboration plan to their institutional legal teams for review.[15][17][18][19] Institutional review on both sides entailed several revisions and negotiations, which ultimately led to the development of a formal data use agreement that governs the CC-PBRN. It is important to note that this is an iterative process requiring open and clear communication from each side. Therefore, after translating the data use agreement into actionable steps, it is revisited regularly by repeating steps one through three in interorganizational team meetings.
Methods
CC-PBRN structure
The CC-PBRN is a centralized repository, which contains de-identified electronic health records (EHR) from the MMTC health system, a private medical Cannabis health system in the state of Florida. FSU-iRACE and MMTC signed data use agreements, which allowed for the transfer of EHR Health Insurance Portability and Accountability Act (HIPAA)–limited data sets to the data trust repository. Data elements from the EHR include diagnoses, procedures, place of service, vital signs, and patient-reported outcomes. The CC-PBRN data team has considerable experience with data standards and LOINC (Logical Observation Identifiers Names and Codes), which are used to ensure that the data are well-organized for research purposes. Data was downloaded from the MMTC EHR July 2021, and final analyses of that data, reported here, were completed November 2021. All currently active patients (n = 43,802) with a certified qualifying medical condition were included in analyses. The CC-PBRN data protocol is approved by the Florida State University Institutional Review Board (FSU IRB).
Florida medical marijuana laws, qualifying conditions, and the process of patient certification
To be certified for medical marijuana in the state of Florida, patients must schedule an appointment with a qualified medical marijuana physician to receive certification of eligibility for a medical marijuana card. In Florida, patients must pay for a physician to certify them, which is approximately $150-$200 depending on the physician. This cost is not covered by any insurance. Once the patient receives certification from a qualified physician, they must use the state portal to apply online for their medical marijuana use registry identification card. Patients also have to pay the state $75, a license fee, to obtain the card. This fee must be paid to the state yearly.
Patients can only be certified under one diagnosis at a time in Florida. Therefore, while other qualifying conditions can be noted in a patient’s chart, physicians must clearly document which one specific qualifying condition they are certifying the patient under. Patients must be certified by a medical marijuana physician every 210 days in order to be an active medical marijuana patient. The state of Florida has a limited number of conditions for which an individual can be certified to obtain a medical marijuana card. Only those diagnosed by a medical doctor with one of the following conditions are eligible for registration in Florida: (a) cancer; (b) epilepsy; (c) glaucoma; (d) positive status for human immunodeficiency virus (HIV); (e) acquired immune deficiency syndrome (AIDS); (f) post-traumatic stress disorder (PTSD); (g) amyotrophic lateral sclerosis; (h) Crohn’s disease; (i) Parkinson’s disease; (j) and multiple sclerosis.
It is important to note that in the state of Florida only medical doctors who have obtained an additional qualifying license with the state of Florida to certify patients for medical marijuana can do so. Therefore, patients who have been diagnosed by their treating physicians must take their medical records to a qualified medical marijuana physician who has the license to certify them for medical marijuana. When a patient does not have one of the conditions listed above—for example they have sickle cell or lupus or anxiety, or any other condition not on the list—if their symptoms are severe enough and can be marked as comparable to one of the conditions on the list, then they can be diagnosed under what is called option (k), "medical conditions of the same kind or class as or comparable to those enumerated in paragraphs (a)-(j)."
Beyond categories (a)-(k), there are two additional unique categories in the state of Florida, noted in the law as (l) and (m), that qualify for certification. For category (l), advocates for those with a terminal condition requested their own category for certification so we now have category (l) "a terminal condition diagnosed by a physician other than the qualified physician issuing the physician certification." Condition (m) “chronic nonmalignant pain” is the most confusing condition in Florida law due to the very narrow definition of the condition. Per Florida law “chronic nonmalignant pain” means “pain that is caused by a qualifying medical condition or that originates from a qualifying medical condition and persists beyond the usual course of that qualifying medical condition.” An example of who fits in that condition is someone who has been cured of cancer and they can no longer be certified under a cancer diagnosis. The law now allows for them to be certified under “chronic nonmalignant pain” because even though they were originally certified due to having cancer, once they are cured (the malignant cancer is gone), if pain still exists possibly from treatment in anyway, the patient will still qualify for a medical marijuana card under the chronic nonmalignant pain condition now that they are cancer-free. Therefore, if someone did not have any of the diagnoses of (a)-(j) and recovered from the diagnosis, then they cannot be certified under condition (m). See Florida Medical Marijuana state statue 381.896 subsection 2 for a more detailed explanation of how Florida defines qualifying medical conditions.
Definition of relevant variables
Qualifying medical conditions: (a) cancer; (b) epilepsy; (c) glaucoma; (d) positive status for HIV; (e) AIDS; (f) PTSD; (g) amyotrophic lateral sclerosis; (h) Crohn’s disease; (i) Parkinson’s disease; (j) and multiple sclerosis.
Comparable medical conditions: Medical conditions of the same kind or class as or comparable to those enumerated in (a)-(j) above.
Symptom reduction: This was assessed using data obtained from the EHR during the follow-up patient visit. We chose this measure because it is captured at each patient visit and was readily available in the EHR. The single item used is not a standard validated measure and it did not ask about specific symptoms such as sleep, pain, or mood. Instead it was a single item global patient reported outcome: "Indicate the percent reduction in severity of symptoms since your initial visit by selecting one of the following options: No percent; 10%; 20%; 30%; 40%; 50%; 60%; 70%; 80%; 90%; 100%." We assessed the percentage of decrease or no change in symptomatology patients reported at their first recertification visit. The first recertification visit generally occurs 210 days after their initial certification.
Data management
In accordance with the FSU IRB, all data collected from MMTC is de-identified, such that no protected health information (PHI) or identifying information is utilized by end-users. De-identified data is stored in the CC-PBRN database, which is maintained on secure servers managed by Florida State University’s College of Medicine Information Technology department. After initial data cleaning and recoding by the data team, a completely de-identified dataset is created to allow researchers to analyze the data. To encourage others to use the data, we created a formal concept system that researchers can use to request a de-identified dataset.
Data analysis
All analyses were conducted using SAS Version 9.4 (Cary, NC).[20] Tabulated descriptive statistics were computed to examine sample characteristics, including socio-demographics, history of substance use, qualifying medical conditions, and number of certifications and recertifications. The sample was stratified based on qualifying medical condition. This was done because in Florida patients are certified for one of 13 qualifying medical condition, one of which is a roll-up category named “comparable medical condition.” A comparable medical condition is defined as “a medical condition of the same kind or class as or comparable to cancer, epilepsy, glaucoma, HIV, AIDS, posttraumatic stress disorder, ALS, Crohn’s disease, Parkinson’s disease, or multiple sclerosis.” Certifications can also be issued for a terminal condition diagnosed by a physician or chronic non-malignant pain which is narrowly defined as pain that is caused by a qualifying medical condition or that originates from a qualifying medical condition and persists beyond the usual course. We grouped those certified under a comparable medical condition in one group, and all other qualifying medical conditions (ALS, cancer, HIV/AIDS, etc.) were collapsed into a separate group. Chi-Square or t-test analyses when applicable were used to assess potential differences in sample characteristics.
In regards to missing data, all data for this paper were reported as is, including variables with missingness which include race and gender. Missingness is identified in the sociodemographic table as unknown.
Results
The CC-PBRN currently has 43,802 active patients (see Table 1). There are 24 clinics in the network, with nine in North Florida, 11 in Central Florida, and four in South Florida. The majority (73.1%) of patients in the network were certified for a comparable medical condition. PTSD was the second most common qualifying medical condition (12%), followed by cancer (8%).
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
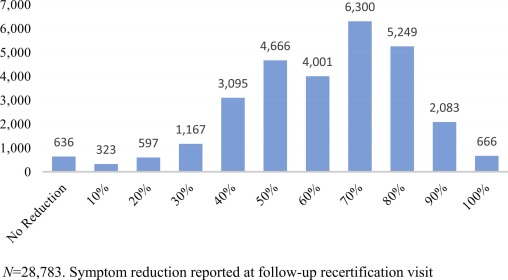
Table 1 shows the summary demographic statistics for the entire sample and by qualifying medical condition. The mean age for the CC-PBRN sample is 51.5 years (SD 16.2). 50.07% are female, and a majority (49.07%) identify as white, with approximately four percent identifying as Hispanic/Latinx. Forty-three percent of the sample report a history of alcohol use and 21% report a history of tobacco use. 34.7% of the sample had only an initial certification, with 35.6% having one to two recertifications, and 29.6% with three or more recertifications. Of the 28,783 with recertification data, 28% (n = 7,998)) report an 80% or greater reduction in symptoms (see Fig. 1). Chi-square analyses show that men are less likely to be certified under a comparable condition, and those certified under a comparable condition are more likely to report a history of alcohol use.
|
Discussion
CC-PBRN is a practice-based research network in Florida with the overarching goal of uniting researchers, physicians, patients, and stakeholders in Florida to address critical gaps in Cannabis science knowledge and other complementary care approaches. This network serves as a statewide resource for patient-reported outcomes research in medical marijuana. The CC-PBRN is an academic-industry partnership between FSU-iRACE and MMTC, and the partnership provides the infrastructure to do bio-behavioral complementary care research across a wide variety of complementary approaches with populations in need of these services. The network currently has numerous outpatient practices with thousands of patients that are a potential source of research participants for both observational studies as well as traditional clinical trials. The outpatient clinical practices can also serve as units of analysis for pragmatic trials comparing different care protocols and organizational structures.
Challenges identified and addressed
As noted earlier, due to the differing foci of academic and business organizations, there were key challenges that had to be negotiated. The first challenge identified was the concern from MMTC that their proprietary information and process would be publicly disclosed, giving similar businesses a competitive advantage or limit their advantage in the marketplace.[21] To address this, we engaged an innovation-focused approach to the research such that the mission of the partnership was to advance complementary care and Cannabis science in the state of Florida. The new knowledge would be shared across patient populations, providers, researchers, and policymakers. All knowledge gained benefits both organizations. Further, we work to identify how new knowledge could improve patient care and create new evidence-based practices across the organization. Through this process we developed a working relationship between the organization in which the internal business agenda does not compete with the mission of the CC-PBRN.
The second challenge identified was academic freedom within the research agenda. Researchers on the FSU-iRACE side insisted on academic freedom since often the problems prioritized as most urgent by those in the industry differ from those in academia. It is difficult to find a common issue that both academia and industry have prioritized. Therefore, we identified the resources of the partnership as a way to define clear expertise in certain domains. FSU-iRACE had the academic expertise and skills needed to perform data management activities and implement the research plan, while MMTC had the data, patients, and providers needed to answer the research questions of interest.[1][22] While there are very few examples of how to negotiate the challenges presented by business-defined priorities versus academic research agendas, reliance on the initial SWOT enabled the partnership to move forward and the building of a viable data use agreement.
The third and final concerned how we would distribute the knowledge outputs generated by the CC-PBRN. Given that business practices generally do not align with the practice of open science, the primary model in academia, we had to identify how business assets would be protected but not interfere with the integrity of the scientific process. In the FSU-iRACE-MMTC collaboration, we had to address that the knowledge outputs created through the partnership would not have an inherent absolute dollar value return on investment (ROI), but instead the added value added would be in how the knowledge could be used to serve the constituents of both organizations. Additionally, the partnership provided an opportunity to advance medical Cannabis science, which is limited in the state of Florida[23][24], and the available expertise to accomplish this task were too important to ignore. We used initial meetings to discuss common practices and expectations around knowledge output dissemination. We identified common intellectual priorities that were independent of business practices, and we created safeguards such as de-identified data, aggregated results, data transformation for scientific purposes, and assurance of academic freedom to ensure integrity in the process. Ownership of the intellectual property was clearly spelled out in the data use agreement, given that the majority of the research on academic-industry partnerships identify that control and ownership of the intellectual property created through the partnership is often the greatest challenge.[21]
In recent years, the potential efficacy of Cannabis as a medical product has generated a great deal of political discourse in the United States and has resulted in pursuits to legalize the use of medical marijuana. Currently 36 states and four territories allow for the medical use of Cannabis products. In Florida, medical marijuana was approved for use in January 2017.[25] The state constitution and Florida statutes specify debilitating conditions eligible for medical marijuana treatment prescribed by a qualified physician, as noted prior. However, Florida faces significant gaps in the scientific knowledge needed to manage medical marijuana care due to the limited research that has been conducted in this area[26][27], and specifically the lack of research on patient-reported outcomes (PRO) of use.[23][24] The U.S. Food and Drug Administration (FDA) defines a PRO as any report of the status of a patient’s health condition that comes directly from the patient, without interpretation of the patient’s response by a clinician or anyone else.[28] For optimal results in healthcare, patient input is needed.[29][30][31][32][33][34] Doctors, researchers, and policymakers need to know what patients feel and perceive about the effects of treatment and services. The CC-PBRN serves as an incubator for PRO research in Florida and throughout the US.
Strengths and limitations
While the CC-PBRN has numerous strengths, there are several limitations. First, the current EHR system does not force responses for certain demographic data. That data is given voluntarily by patients; therefore, we currently have incomplete demographic data for race, education, and income level. Second, for those certified under a comparable condition, we can only approximate symptoms being treated to the nearest condition allowed by law, which may not reflect all conditions experienced by patients. Third, data regarding other chronic conditions is all self-reported, and patients may over-report conditions that have not been diagnosed by a physician. Finally, symptom reduction was not measured using a validated symptom reduction measure. Future work should incorporate validated measures of symptom reduction.
Despite these limitations, the CC-PBRN is a vital research network that provides numerous resources for the advancement of complementary care and Cannabis science. CC-PBRN is a representative sample of medical marijuana users in the state of Florida[23] and offers investigators the opportunity to engage in research with ethnically, racially, and geographically (rural, urban, and suburban) diverse populations across the state of Florida. Research queries can be conducted using de-identified patient records, which assists with hypothesis generation and study feasibility estimations. It also provides support services for implementing grant-funded research in a real-world practice-based setting.
Conclusion
We described the development of an academic-industry partnership to improve Cannabis science, presented descriptive data on the research network, and described the challenges of developing an effective academic-industry partnership. The results of this work demonstrate that academic-industry partnerships that serve the common good are possible with collaboration, communication, and cooperation of all organizations involved. The CC-PBRN is poised as the catalyst for medical marijuana research in the state of Florida. Given the diversity of the populations and chronic conditions in the network, it will also support patient reported outcomes research across a host of complementary care approaches. The data generated by the CC-PBRN can be used to inform and guide patient-centered care, clinical decision-making, and health policy decisions. These data are an important component in learning healthcare systems.
Sawatzky et al.[29] identified nine types of potential impacts PRO trials can have; they include informing clinical practice, informing clinical guidelines, informing health policy, supporting drug approval, supporting pricing and supporting reimbursement decisions, informing clinical and shared decision-making, and informing consent for treatment. Given the number of patients certified for medical marijuana in Florida and the gaps in science, with its focus on complementary care and Cannabis science, the CC-PBRN is poised to make a significant scientific contribution.
Supplementary material
Acknowledgements
Author contributions
All authors made substantive contributions to the development, design, and writing of this manuscript. Dr. Ennis led the development, conceptualization, data analysis plan, interpretation of findings, and writing of the manuscript. Dr. Vance provided clarification on the meaning of raw data fields, interpretation of analyses, and contributed to the writing, and Mr. Bradbury was responsible for all data management (the data structure was transformed from the EMR and managed in SAS), completion of all data analyses, and contributed to interpretation of data and writing of the manuscript.
Funding
This work was supported by pilot funding awarded to Dr. Nicole Ennis from Florida State University-College of Medicine, Florida, USA under award #208106210. As stated in the Data Use Agreement, MMTC allowed FSU to download data directly from their EMR but did not contribute any funding for any of part the work reported in this paper.
Ethical approval
IRB approval for this study was obtained via Florida State University Institutional Review Board.
Consent to participate
Appropriate consent to use the de-identified EHR data in this manuscript was obtained.
Conflict of interest
Nicole Ennis, PhD and Russell Bradbury, MS have no conflict of interest to report. Cameron Vance, PharmD is a co-founder of MMTC Florida.
References
- ↑ 1.0 1.1 1.2 1.3 King, Gary; Persily, Nathaniel (1 October 2020). "A New Model for Industry–Academic Partnerships" (in en). PS: Political Science & Politics 53 (4): 703–709. doi:10.1017/S1049096519001021. ISSN 1049-0965. https://www.cambridge.org/core/product/identifier/S1049096519001021/type/journal_article.
- ↑ Ankrah, Samuel; AL-Tabbaa, Omar (1 September 2015). "Universities–industry collaboration: A systematic review" (in en). Scandinavian Journal of Management 31 (3): 387–408. doi:10.1016/j.scaman.2015.02.003. https://linkinghub.elsevier.com/retrieve/pii/S0956522115000238.
- ↑ Perkmann, Markus; Schildt, Henri (1 June 2015). "Open data partnerships between firms and universities: The role of boundary organizations" (in en). Research Policy 44 (5): 1133–1143. doi:10.1016/j.respol.2014.12.006. https://linkinghub.elsevier.com/retrieve/pii/S0048733314002248.
- ↑ D’Este, Pablo; Perkmann, Markus (1 June 2011). "Why do academics engage with industry? The entrepreneurial university and individual motivations" (in en). The Journal of Technology Transfer 36 (3): 316–339. doi:10.1007/s10961-010-9153-z. ISSN 0892-9912. http://link.springer.com/10.1007/s10961-010-9153-z.
- ↑ Agency for Healthcare Research and Quality (October 2018). "Primary Care Practice-Based Research Networks". Agency for Healthcare Research and Quality. https://www.ahrq.gov/research/findings/factsheets/primary/pbrn/index.html.
- ↑ Lee, Hyangsook; Peng, Wenbo; Steel, Amie; Reid, Rebecca; Sibbritt, David; Adams, Jon (1 April 2019). "Complementary and alternative medicine research in practice-based research networks: A critical review" (in en). Complementary Therapies in Medicine 43: 7–19. doi:10.1016/j.ctim.2018.12.023. https://linkinghub.elsevier.com/retrieve/pii/S0965229918307131.
- ↑ Cohen, Deborah; Keller; DeVoe; Davis (1 September 2012). "Characteristics and lessons learned from practice-based research networks (PBRNs) in the United States" (in en). Journal of Healthcare Leadership: 107. doi:10.2147/JHL.S16441. ISSN 1179-3201. PMC PMC4512302. PMID 26213481. http://www.dovepress.com/characteristics-and-lessons-learned-from-practice-based-research-netwo-peer-reviewed-article-JHL.
- ↑ Carey, T. S.; Halladay, J. R.; Donahue, K. E.; Cykert, S. (1 September 2015). "Practice-based Research Networks (PBRNs) in the Era of Integrated Delivery Systems" (in en). The Journal of the American Board of Family Medicine 28 (5): 658–662. doi:10.3122/jabfm.2015.05.140353. ISSN 1557-2625. PMC PMC5287053. PMID 26355138. http://www.jabfm.org/cgi/doi/10.3122/jabfm.2015.05.140353.
- ↑ Riley-Behringer, Maureen; Davis, Melinda M.; Werner, James J.; Fagnan, L. J.; Stange, Kurt C. (1 October 2017). "The evolving collaborative relationship between Practice-Based Research Networks (PBRNs) and Clinical and Translational Science Awardees (CTSAs)" (in en). Journal of Clinical and Translational Science 1 (5): 301–309. doi:10.1017/cts.2017.305. ISSN 2059-8661. PMC PMC5828176. PMID 29503735. https://www.cambridge.org/core/product/identifier/S2059866117003053/type/journal_article.
- ↑ 10.0 10.1 Castañer, Xavier; Oliveira, Nuno (1 July 2020). "Collaboration, Coordination, and Cooperation Among Organizations: Establishing the Distinctive Meanings of These Terms Through a Systematic Literature Review" (in en). Journal of Management 46 (6): 965–1001. doi:10.1177/0149206320901565. ISSN 0149-2063. http://journals.sagepub.com/doi/10.1177/0149206320901565.
- ↑ Combs, J.G.; Ketchen Jr., D.J. (1999). "Explaining interfirm cooperation and performance: toward a reconciliation of predictions from the resource-based view and organizational economics". Strategic Management Journal 20 (9): 867–88. doi:10.1002/(SICI)1097-0266(199909)20:9<867::AID-SMJ55>3.0.CO;2-6.
- ↑ Ashkenas, R. (2015). "There is a difference between cooperation and collaboration". Harvard Business Review 20: 1–3.
- ↑ Casebeer, A. (1993 Mar 17-Apr 6). "Application of SWOT analysis". British Journal of Hospital Medicine 49 (6): 430–431. ISSN 0007-1064. PMID 8472105. https://pubmed.ncbi.nlm.nih.gov/8472105.
- ↑ Castañer, Xavier; Ketokivi, Mikko (10 December 2018), Joseph, John; Baumann, Oliver; Burton, Richard et al.., eds., "Toward a Theory of Organizational Integration" (in en), Advances in Strategic Management (Emerald Publishing Limited) 40: 53–80, doi:10.1108/s0742-332220180000040002, ISBN 978-1-78756-330-8, https://www.emerald.com/insight/content/doi/10.1108/S0742-332220180000040002/full/html
- ↑ 15.0 15.1 Gulati, Ranjay; Singh, Harbir (1 December 1998). "The Architecture of Cooperation: Managing Coordination Costs and Appropriation Concerns in Strategic Alliances". Administrative Science Quarterly 43 (4): 781. doi:10.2307/2393616. https://www.jstor.org/stable/2393616?origin=crossref.
- ↑ Gulati, Ranjay; Wohlgezogen, Franz; Zhelyazkov, Pavel (1 June 2012). "The Two Facets of Collaboration: Cooperation and Coordination in Strategic Alliances" (in en). Academy of Management Annals 6 (1): 531–583. doi:10.5465/19416520.2012.691646. ISSN 1941-6520. http://journals.aom.org/doi/10.5465/19416520.2012.691646.
- ↑ Gong, Yaping; Shenkar, Oded; Luo, Yadong; Nyaw, Mee-Kau (1 October 2007). "Do multiple parents help or hinder international joint venture performance? The mediating roles of contract completeness and partner cooperation" (in en). Strategic Management Journal 28 (10): 1021–1034. doi:10.1002/smj.626. https://onlinelibrary.wiley.com/doi/10.1002/smj.626.
- ↑ Hoetker, Glenn; Mellewigt, Thomas (1 October 2009). "Choice and performance of governance mechanisms: matching alliance governance to asset type" (in en). Strategic Management Journal 30 (10): 1025–1044. doi:10.1002/smj.775. https://onlinelibrary.wiley.com/doi/10.1002/smj.775.
- ↑ Hoffmann, Werner; Lavie, Dovev; Reuer, Jeffrey J.; Shipilov, Andrew (1 December 2018). "The interplay of competition and cooperation" (in en). Strategic Management Journal 39 (12): 3033–3052. doi:10.1002/smj.2965. https://onlinelibrary.wiley.com/doi/10.1002/smj.2965.
- ↑ "SAS 9.4 — Today and Tomorrow". SAS Support. SAS Institute, Inc. https://support.sas.com/software/94/.
- ↑ 21.0 21.1 Ahuja, Gautam; Lampert, Curba Morris; Novelli, Elena (1 April 2013). "The Second Face of Appropriability: Generative Appropriability and Its Determinants" (in en). Academy of Management Review 38 (2): 248–269. doi:10.5465/amr.2010.0290. ISSN 0363-7425. http://journals.aom.org/doi/10.5465/amr.2010.0290.
- ↑ Jasny, B. R.; Wigginton, N.; McNutt, M.; Bubela, T.; Buck, S.; Cook-Deegan, R.; Gardner, T.; Hanson, B. et al. (25 August 2017). "Fostering reproducibility in industry-academia research" (in en). Science 357 (6353): 759–761. doi:10.1126/science.aan4906. ISSN 0036-8075. https://www.sciencemag.org/lookup/doi/10.1126/science.aan4906.
- ↑ 23.0 23.1 23.2 Costales, Brianna; van Boemmel-Wegmann, Sascha; Winterstein, Almut; Segal, Richard (27 May 2021). "Clinical Conditions and Prescription Drug Utilization among Early Medical Marijuana Registrants in Florida" (in en). Journal of Psychoactive Drugs 53 (3): 185–194. doi:10.1080/02791072.2020.1864069. ISSN 0279-1072. https://www.tandfonline.com/doi/full/10.1080/02791072.2020.1864069.
- ↑ 24.0 24.1 Howell, Khadesia; Washington, Alexandria; Williams, Paula M.; Mathis, Arlesia L.; Luque, John S. (2019). "Medical Marijuana Policy Reform Reaches Florida: A Scoping Review". Florida Public Health Review 16: 128–136. PMC 6936729. PMID 31891164. https://pubmed.ncbi.nlm.nih.gov/31891164.
- ↑ "About the OMMU". Office of Medical Marijuana Use. 2018. https://knowthefactsmmj.com/about/.
- ↑ Petrie, Gavin N.; Nastase, Andrei S.; Aukema, Robert J.; Hill, Matthew N. (1 September 2021). "Endocannabinoids, cannabinoids and the regulation of anxiety". Neuropharmacology 195: 108626. doi:10.1016/j.neuropharm.2021.108626. ISSN 1873-7064. PMID 34116110. https://pubmed.ncbi.nlm.nih.gov/34116110.
- ↑ National Academies of Sciences, Engineering, and Medicine (U.S.), ed. (2017). The health effects of cannabis and cannabinoids: the current state of evidence and recommendations for research. Washington, DC: The National Academies Press. ISBN 978-0-309-45304-2. OCLC 984512600. https://www.worldcat.org/title/mediawiki/oclc/984512600.
- ↑ U.S. Department of Health and Human Services (December 2009). "Guidance for Industry - Patient-Reported Outcome Measures: Use in Medical Product Development to Support Labeling Claims" (PDF). Food and Drug Administration. https://www.fda.gov/media/77832/download.
- ↑ 29.0 29.1 Sawatzky, Richard; Kwon, Jae-Yung; Barclay, Ruth; Chauhan, Cynthia; Frank, Lori; van den Hout, Wilbert B.; Nielsen, Lene Kongsgaard; Nolte, Sandra et al. (1 December 2021). "Implications of response shift for micro-, meso-, and macro-level healthcare decision-making using results of patient-reported outcome measures" (in en). Quality of Life Research 30 (12): 3343–3357. doi:10.1007/s11136-021-02766-9. ISSN 0962-9343. PMC PMC8602130. PMID 33651278. https://link.springer.com/10.1007/s11136-021-02766-9.
- ↑ Field, Jonathan; Holmes, Michelle M.; Newell, Dave (29 July 2019). "PROMs data: can it be used to make decisions for individual patients? A narrative review" (in English). Patient Related Outcome Measures 10: 233–241. doi:10.2147/PROM.S156291. PMC PMC6681163. PMID 31534379. https://www.dovepress.com/proms-data-can-it-be-used-to-make-decisions-for-individual-patients-a--peer-reviewed-fulltext-article-PROM.
- ↑ Lai, Cara H.; Shapiro, Lauren M.; Amanatullah, Derek F.; Chou, Loretta B.; Gardner, Michael J.; Hu, Serena S.; Safran, Marc R.; Kamal, Robin N. (1 April 2022). "A framework to make PROMs relevant to patients: qualitative study of communication preferences of PROMs" (in en). Quality of Life Research 31 (4): 1093–1103. doi:10.1007/s11136-021-02972-5. ISSN 0962-9343. https://link.springer.com/10.1007/s11136-021-02972-5.
- ↑ Zidarov, Diana; Zidarova-Carrié, Alexia; Visca, Regina; Miller, J. Marc; Brecht, Krista; Viens, Natacha; Ahmed, Sara (1 July 2020). "Core patient-reported outcome domains for routine clinical care in chronic pain management: patients’ and healthcare professionals’ perspective" (in en). Quality of Life Research 29 (7): 2007–2020. doi:10.1007/s11136-020-02459-9. ISSN 0962-9343. http://link.springer.com/10.1007/s11136-020-02459-9.
- ↑ Calvert, Melanie; Kyte, Derek; Price, Gary; Valderas, Jose M; Hjollund, Niels Henrik (24 January 2019). "Maximising the impact of patient reported outcome assessment for patients and society" (in en). BMJ: k5267. doi:10.1136/bmj.k5267. ISSN 0959-8138. https://www.bmj.com/lookup/doi/10.1136/bmj.k5267.
- ↑ Holmes, Michelle M.; Lewith, George; Newell, David; Field, Jonathan; Bishop, Felicity L. (1 February 2017). "The impact of patient-reported outcome measures in clinical practice for pain: a systematic review" (in en). Quality of Life Research 26 (2): 245–257. doi:10.1007/s11136-016-1449-5. ISSN 0962-9343. PMC PMC5288411. PMID 27815820. http://link.springer.com/10.1007/s11136-016-1449-5.
Notes
This presentation is faithful to the original, with only a few minor changes to presentation. Some grammar and punctuation was cleaned up to improve readability. In some cases important information was missing from the references, and that information was added. No other information was changed, in accordance with the "No Derivatives" portion of the license.