Journal:Combined ambient ionization mass spectrometric and chemometric approach for the differentiation of hemp and marijuana varieties of Cannabis sativa
| Full article title | Combined ambient ionization mass spectrometric and chemometric approach for the differentiation of hemp and marijuana varieties of Cannabis sativa |
|---|---|
| Journal | Journal of Cannabis Research |
| Author(s) | Chambers, Megan I.; Beyramysoltan, Samira; Garosi, Benedetta; Musah, Rabi A. |
| Author affiliation(s) | State University of New York |
| Primary contact | Email: rmusah at albany dot edu |
| Year published | 2023 |
| Volume and issue | 5 |
| Article # | 5 |
| DOI | 10.1186/s42238-023-00173-0 |
| ISSN | 2522-5782 |
| Distribution license | Creative Commons Attribution 4.0 International |
| Website | https://jcannabisresearch.biomedcentral.com/articles/10.1186/s42238-023-00173-0 |
| Download | https://jcannabisresearch.biomedcentral.com/counter/pdf/10.1186/s42238-023-00173-0.pdf (PDF) |
|
|
This article should be considered a work in progress and incomplete. Consider this article incomplete until this notice is removed. |
Abstract
Background: Hemp and marijuana are the two major varieties of Cannabis sativa. While both contain Δ9-tetrahydrocannabinol (THC), the primary psychoactive component of C. sativa, they differ in the amount of THC that they contain. Presently, U.S. federal laws stipulate that C. sativa containing greater than 0.3% THC is classified as marijuana, while plant material that contains less than or equal to 0.3% THC is hemp. Current methods to determine THC content are chromatography-based, which requires extensive sample preparation to render the materials into extracts suitable for sample injection, for complete separation and differentiation of THC from all other analytes present. This can create problems for forensic laboratories due to the increased workload associated with the need to analyze and quantify THC in all C. sativa materials.
Method: The work presented herein combines direct analysis in real time high-resolution mass spectrometry (DART-HRMS) and advanced chemometrics to differentiate hemp and marijuana plant materials. Samples were obtained from several sources (e.g., commercial vendors, DEA-registered suppliers, and the recreational Cannabis market). DART-HRMS enabled the interrogation of plant materials with no sample pretreatment. Advanced multivariate data analysis approaches, including random forest and principal component analysis (PCA), were used to optimally differentiate these two varieties with a high level of accuracy.
Results: When PCA was applied to the hemp and marijuana data, distinct clustering that enabled their differentiation was observed. Furthermore, within the marijuana class, subclusters between recreational and DEA-supplied marijuana samples were observed. A separate investigation using the silhouette width index to determine the optimal number of clusters for the marijuana and hemp data revealed this number to be two. Internal validation of the model using random forest demonstrated an accuracy of 98%, while external validation samples were classified with 100% accuracy.
Discussion: The results show that the developed approach would significantly aid in the analysis and differentiation of C. sativa plant materials prior to launching painstaking confirmatory testing using chromatography. However, to maintain and/or enhance the accuracy of the prediction model and keep it from becoming outdated, it will be necessary to continue to expand it to include mass spectral data representative of emerging hemp and marijuana strains/cultivars.
Keywords: Cannabis sativa, ambient ionization mass spectrometry, direct analysis in real time—high-resolution mass spectrometry, multivariate data analysis, random forest, principal component analysis
Background
Among the greatest challenges to emerge for U.S. forensic laboratories in recent years are those attributed to the increased legalization and decriminalization of marijuana at the state level, in addition to the permitted production of hemp. The 2019 National Institute of Justice (NIJ) Report to Congress: Needs Assessment of Forensic Laboratories and Medical Examiner/Coroner Offices identified this area as requiring focused attention towards improving criminal justice practices in the USA. [NIJ 2019] The challenge that hemp and marijuana present is as follows: both are major varieties of the same species Cannabis sativa, often referred to as Cannabis. While they each contain Δ9-tetrahydrocannabinol (THC), which is the primary psychoactive component of C. sativa, marijuana and hemp differ in the amount of this molecule that is present. In 2018, the U.S. federal guidelines stipulated that C. sativa which contains greater than 0.3% THC is a scheduled controlled substance (i.e., marijuana), while plant material that contains less than or equal to 0.3% is a legal agricultural commodity (i.e., hemp). [H.R.2 – 115th Congress 2017–2018] This definition has imposed severe challenges on crime labs. Among them is the dramatic increase in workload that results from the need to analyze and quantify the THC content of all C. sativa samples so that seized material can be appropriately designated. This is a time-consuming and resource-intensive enterprise that to greater and greater extents is consuming even larger forensic lab resources. Furthermore, defining the error cutoff for the 0.3% designation presents a challenge for the analysis of samples whose THC level is at the threshold.
Traditionally, hemp and marijuana plant materials are differentiated by determining the THC content through chromatography-based approaches such as gas chromatography-flame ionization detection (GC-FID) and gas chromatography-mass spectrometry (GC–MS) [Pourseyed Lazarjani et al. 2020], in addition to high-performance liquid chromatography (HPLC) coupled to ultraviolet (UV) detection. [UNODC 2009] However, to accurately determine the THC content with these approaches, THC must be separated from all other components in the material (i.e., cannabinoids, terpenes, etc.) prior to quantification. One way to achieve this is to extend run times to allow for baseline separation between cannabinoids and other analytes present. Another option is to introduce a chemical derivatization step into the sample preparation protocol (which can be time-consuming), to differentiate between cannabinoids and their corresponding cannabinoid acids (e.g., THC and tetrahydrocannabinolic acid [THCA]). Although many investigations have been successful at differentiating between hemp and marijuana varieties or strains [Wiebelhaus et al. 2016; Horne et al. 2020; Pacula et al. 2016; Fischedick et al. 2010], the methods are reliant upon chromatography and are therefore susceptible to the aforementioned delineated challenges that can arise using this technique (i.e., lengthy run times, column contamination, etc.). Research towards developing, optimizing, and validating methods suitable for field testing of Cannabis materials has also been investigated.
Colorimetric tests represent a large percentage of these methods, which yield a presumptive result (by producing a color change) [Alonzo et al. 2018] when Cannabis-related substances are present, without the need for additional instrumentation (i.e., it is visible to the naked eye). Some examples include the 4-aminophenol test [Lewis et al. 2021; Acosta et al. 2022], Fast Blue BB test [Acosta et al. 2022; Acosta and Almirall 2021], and Duquenois-Levine test. [Forrester 1997] Similar to chromatography-based methods, these tests all rely upon the detection of THC specifically, which can complicate analyses because both marijuana and hemp contain this compound. Thus, while the distinction between marijuana and hemp has been defined based on THC levels, this is accompanied by several analytical challenges (i.e., baseline separation of molecules by chromatography-based methods, lengthy sample preparation protocols, and presumptive tests that can yield false positives [Gabrielson et al. 2016], etc.).
An alternative less arbitrary approach is to base the distinction between them on the genome-defined differences in their metabolome signatures (i.e., small-molecule profiles). Studies utilizing the genetic profiles of Cannabis, such as genotyping-by-sequencing (GBS) and single-nucleotide polymorphisms (SNPs), have shown that, although they represent the same species, hemp and marijuana differ at the genome-wide level. [Sawler et al. 2015; Roman et al. 2020; Schwabe et al. 2021] However, in addition to the fact that many crime laboratories are not positioned to integrate these types of analyses into current workflows, one of the bottlenecks to the routine use of the genome-defined small-molecule profiles for species attribution is the challenge of accessing this information quickly and reliably. One way to rapidly reveal this information, and subsequently distinguish between hemp and marijuana, is to combine an ambient ionization mass spectrometric technique—e.g., direct analysis in real time high-resolution mass spectrometry (DART-HRMS) [Cody et al. 2005]—with advanced statistical analysis. Ambient ionization methods (e.g., DART-HRMS, desorption electrospray ionization [DESI-MS]) have proven successful at screening for cannabinoids in Cannabis plant materials [Chambers and Musah 2022; Rodriguez-Cruz 2006; Chambers and Musah 2023] and Cannabis-derived products (e.g., edibles, personal-care products, vape products, concentrates). [Chambers and Musah 2022; Chambers and Musah. 2023] The unique capabilities of DART-HRMS are well-suited for the analysis of complex plant materials; the results are characterized by having high chemical information content, and little to no sample preparation prior to interrogating the materials is required. When applied to DART-HRMS-derived spectra, statistical data processing has enabled the successful differentiation of psychoactive plant species [Beyramysoltan et al. 2019] and their headspace chemical signatures. [Appley et al. 2019] A modified version of DART-MS analysis introduced thermal desorption (TD) into the methodology (TD-DART-MS). One study utilized TD-DART-MS data to differentiate four hemp cultivars using PCA and partial least squares discriminant analysis (PLS-DA). [Dong et al. 2019] Another found that the application of statistical analysis to DART-MS data derived from methanolic extracts of hemp and marijuana samples revealed the potential for utilizing this method for optimally differentiating hemp and marijuana varieties. [Pieslak 2021]
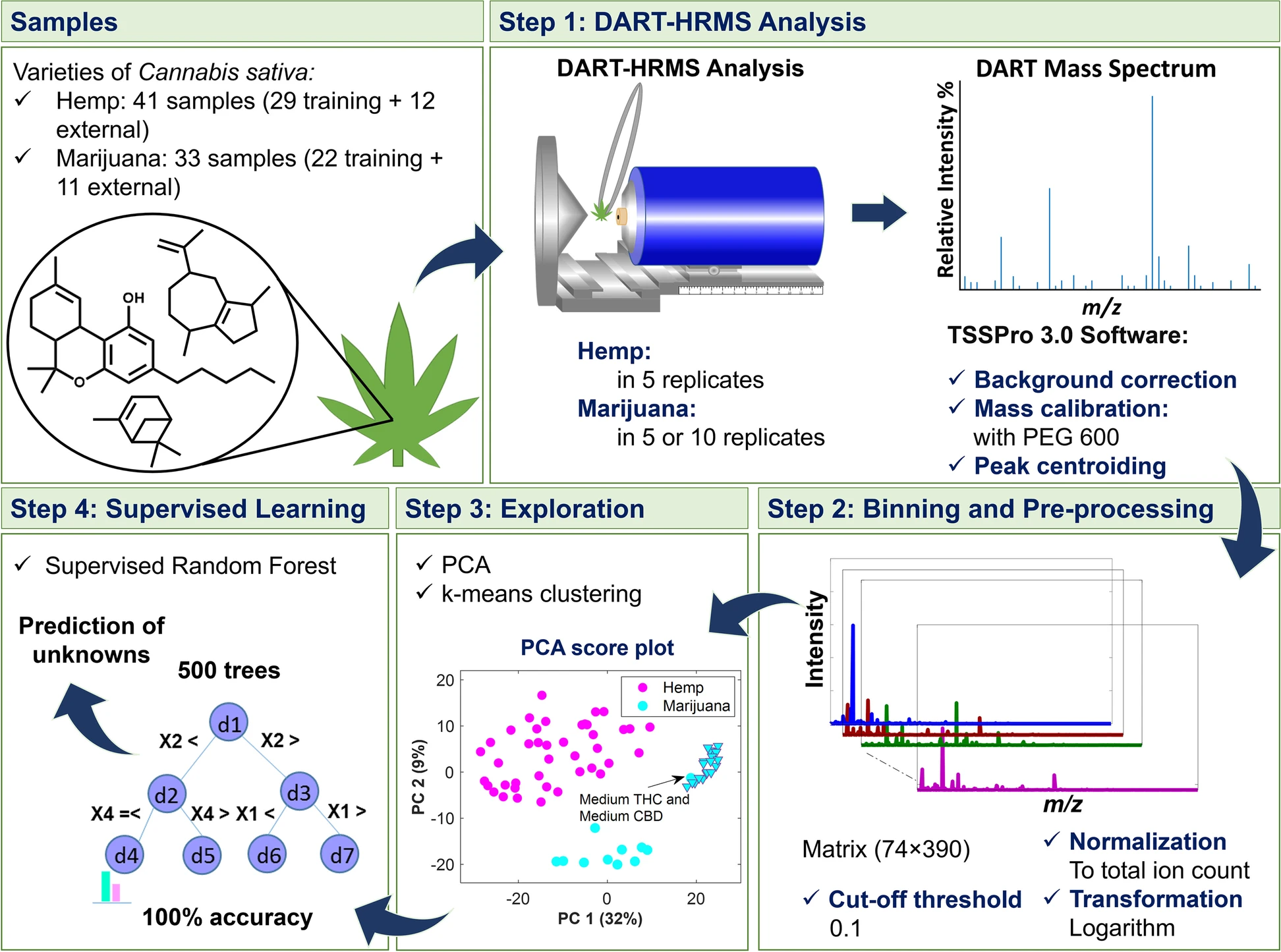
The study presented here, which is summarized in the scheme presented in Fig. 1, utilized DART-HRMS, for the first time, to investigate the complex genome-defined chemical fingerprints of hemp and marijuana (with no sample pretreatment) for the purpose of distinguishing between these two C. sativa varieties using multivariate statistical approaches. Advanced chemometrics was applied to the DART-HRMS data derived from commercial hemp, recreational marijuana, and marijuana samples from Drug Enforcement Administration (DEA)-registered suppliers to develop a robust model by which they (i.e., hemp and marijuana) could be readily differentiated. The success rate of the developed model’s ability to predict external validation samples was 100%, indicating a high level of certainty. Importantly, the developed method circumvents the need to separate and differentiate cannabinoids by chromatography techniques (i.e., the traditional forensic approach for determining the THC concentration in a sample and which is used for differentiating between hemp and marijuana), in addition to bypassing all sample pretreatment steps.
|
Materials and methods
Cannabis sativa plant materials
References
Notes
This presentation is faithful to the original, with only a few minor changes to presentation. Some grammar and punctuation was cleaned up to improve readability. In some cases important information was missing from the references, and that information was added. The original lists references in alphabetical order; they are listed by order of appearance for this version, by design.