Journal:A metabolomics and big data approach to cannabis authenticity (authentomics)
| Full article title | A metabolomics and big data approach to cannabis authenticity (authentomics) |
|---|---|
| Journal | International Journal of Molecular Sciences |
| Author(s) | Jadhav, Pramodkumar D.; Shim, Youn Y.; Paek, Ock J.; Jeon, Jung-Tae; Park, Hyun-Je; Park, Ilbum; Park, Eui-Seong; Kim, Young J.; Reaney, Martin J.T. |
| Author affiliation(s) | University of Saskatchewan, Prairie Tide Diversified, Korea University, Republic of Korea Ministry of Food and Drug Safety, Yuhan Care Company |
| Primary contact | Email: younyoung dot shim at usask dot ca |
| Year published | 2023 |
| Volume and issue | 24(9) |
| Article # | 8202 |
| DOI | 10.3390/ijms24098202 |
| ISSN | 1422-0067 |
| Distribution license | Creative Commons Attribution 4.0 International |
| Website | https://www.mdpi.com/1422-0067/24/9/8202 |
| Download | https://www.mdpi.com/1422-0067/24/9/8202/pdf?version=1683269179 (PDF) |
|
|
This article should be considered a work in progress and incomplete. Consider this article incomplete until this notice is removed. |
Abstract
With the increasing accessibility of cannabis (Cannabis sativa L., also known as marijuana and hemp), its products are being developed as extracts for both recreational and therapeutic use. This has led to increased scrutiny by regulatory bodies, who aim to understand and regulate the complex chemistry of these products to ensure their safety and efficacy. Regulators use targeted analyses to track the concentration of key bioactive metabolites and potentially harmful contaminants, such as heavy metals and other impurities. However, the complexity of cannabis' metabolic pathways requires a more comprehensive approach. A non-targeted metabolomic analysis of cannabis products is necessary to generate data that can be used to determine their authenticity and efficacy. An authentomics approach, which involves combining the non-targeted analysis of new samples with big data comparisons to authenticated historic datasets, provides a robust method for verifying the quality of cannabis products. To meet International Organization for Standardization (ISO) standards, it is necessary to implement authentomics platform technology and build an integrated database of cannabis analytical results. This study is the first to review the topic of the authentomics of cannabis and its potential to meet ISO standards.
Keywords: cannabis authenticity, Cannabis sativa L., authentomics, metabolites, nuclear magnetic resonance
Introduction
Metabolomics is a crucial approach for gaining insight into the largest possible set of low-molecular-weight metabolites present in biological samples. When used in conjunction with genomics, transcriptomics, and proteomics, metabolomics helps shed light on the workings of biological systems as they develop and respond to environmental stimuli.
Metabolomics is downstream of genomics, transcriptomics, and proteomics. [1,2] Comprehensive analysis of the metabolome is predicated on developments in analytical methods, data-handling tools, and database management systems that first generate big data sets using various chemometric techniques and subsequently use multivariate analysis for interpretation. [3] Nuclear magnetic resonance (NMR) and chromatography (gas or liquid) coupled with mass spectrometry (MS) are the most common techniques used in metabolomic analysis.
There are different approaches in metabolomics for the comprehensive analysis of both known and unknown metabolites. One approach, metabolic profiling, involves measuring large sets of metabolites to provide information about metabolism. Such an approach can include the characterization of both metabolites (unknown and known) and metabolic pathways. Analytical methods that focus on the repeated identification and quantification of pre-selected compounds are known as targeted approaches. On the other hand, non-targeted approaches quantify all measurable compounds, regardless of their identification. Both targeted and non-targeted methods can provide information about the concentration of known compounds, while unknown compounds can be interpreted as having relative concentrations.
A third approach, called metabolic fingerprinting, typically generates metabolic information without precise quantification and identification. This latter approach involves the production of a pattern that is, ideally, interpretable. Fingerprinting is used in food or food product authentication, where the fingerprint pattern of the unknown sample is compared with the spectral database of known samples to determine its conformity. [4,5] Metabolic studies can also be classified based on the study objectives, such as (a) informative studies, where the metabolites’ identification and quantification are obtained; (b) discriminative studies, which help to distinguish metabolites among sample populations; and (c) predictive studies, which create statistical models to create class memberships. [6]
Cannabis and its extracts are chemically complex natural mixtures with various biologically active compounds (metabolites). These compounds include phytocannabinoids, terpenoids, flavonoids, nitrogenous compounds, sugars, proteins, fatty acids, and more. (Table 1)
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
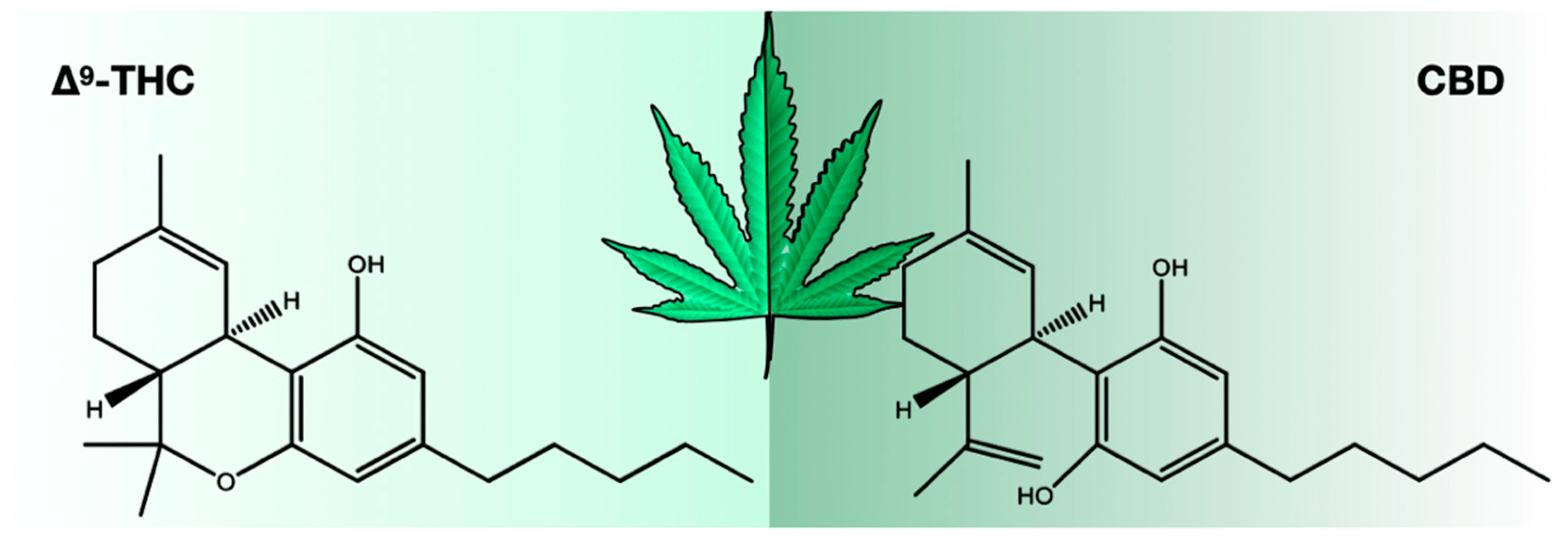
There were 423 compounds reported in the 1980s [7], and in 2017, the number of identified compounds increased to ~565 [11], and more are being identified. The number of cannabinoids detected is now over 120. [8,9,10,11] Among these cannabinoids, psychoactive Δ9-tetrahydrocannabinol (THC) and cannabidiol (CBD) are best known for their contribution to pharmacological activity (Figure 1). These compounds are not the products of metabolic pathways but rather are produced as acidic precursors. The action of heat on these precursors induces decarboxylation and the formation of bioactive compounds. Cannabis extracts contain a range of compounds, including cannabinoids and terpenes, of which many contribute a synergistic “entourage effect” where the therapeutic effect is greater than the sum of the individual compounds. [12] While the U.S. Food and Drug Administration (FDA)-approved drugs such as Epidiolex, Marinol, Syndros, and Cesamet have demonstrated efficacy in treating certain ailments, there is still a great deal of potential for the use of cannabinoids in treating other conditions. Current research suggests that cannabinoids may have anti-inflammatory, analgesic, and anxiolytic properties, among others. However, further clinical trials are needed to fully understand the therapeutic potential of these compounds and mixtures of compounds. Interestingly, cannabinoids bind to receptors and exert biological effects. [13] As one might expect, one class of receptors—cannabinoid receptors—was identified through their interaction with cannabinoids.
|
Metabolomics analysis is useful in studies of plant responses to their environment (e.g., temperature, photoperiod, bioelicitors, fertilizers, water, atmosphere, etc.) and genotypic differences among plants. For example, metabolomics approaches can be used in developing better agronomic practices or the selection of cultivars with superior traits. Gas chromatography (GC) with a flame ionization detector (FID) or mass spectrometer is often used in the analysis of cannabis and cannabis extracts. However, these approaches are limited to measuring metabolites that can be made volatile. Acidic precursors of THC and CBD experience decarboxylation in the typical GC injection port. If this is not carefully controlled, the analysis will be compromised.
Acidic cannabinoids can be stabilized and made more volatile by derivatization, especially via silylation, but the quantification can be less reliable. [14] Moreover, in the high-temperature conditions typical in a GC injector, cannabinoids can thermally oxidize or isomerize. For example, unnatural compounds produced by isomerization, Δ8-THC and cannabinol (CBN), were detected in cannabinoid extract analysis by GC. High-performance liquid chromatography (HPLC) analysis is possible for the non-destructive analysis of cannabinoids, and compounds can be resolved using a range of media, though reverse-phase columns are widely used. While GC methods typically provide better resolution than HPLC, peak overlap in HPLC can typically be overcome when an MS detector is used. When tandem mass spectrometry (MS/MS) is applied, fragmentation patterns can serve to definitively identify cannabinoids. [14] NMR has been used in cannabis extract analysis to discriminate among cultivars and determine the impact of elicitors in cannabis cell suspension cultures. NMR has advantages over chromatographic methods, including simplified sample preparation and non-destructive analysis. These characteristics make some NMR-based analyses suitable for high-throughput analysis and more reproducible than other methods. NMR is much less sensitive than MS but can provide, with fewer sample preparation steps, robust information regarding chemical fingerprints. [15,16] NMR methods also have an additional capability that is not always possible with other methods. NMR methods can be linear over a much larger dynamic range and interference can often be managed. In MS methods, the response is based on ionization phenomena, and the ion suppression of signals is common. Quantitative MS is difficult to accomplish without sophisticated standards for each analyte.
Metabolomic technologies
Many analytical tools have been applied to extract useful metabolomic information. However, due to the chemical heterogeneity of metabolites, large differences in metabolite concentration, and interactions among metabolites, single analytical platforms may fail in determining metabolic profiles. Therefore, combinations of analytical approaches are needed to capture most of the salient information required to characterize complex mixtures of compounds. The selection of the best analytical solution is influenced by the sample matrix, metabolite concentration and properties, and sample amount. Thus, metabolomics is described as an area of science rather than an analytical approach. [17] Metabolomic technologies have been used to identify bioactive compounds in cannabis; they are summarized in Table 2 and briefly described below.
| ||||||||||||||||||||||||||||||||||||||||||||||||
References
Notes
This presentation is faithful to the original, with only a few minor changes to presentation. Some grammar and punctuation was cleaned up to improve readability. In some cases important information was missing from the references, and that information was added.