Journal:Enzyme immunoassay for measuring aflatoxin B1 in legal cannabis
| Full article title | Enzyme immunoassay for measuring aflatoxin B1 in legal cannabis |
|---|---|
| Journal | Toxins |
| Author(s) | Di Nardo, Fabio; Cavalera, Simone; Baggiani, Claudio; Ciarello, Matteo; Pazzi, Marco; Anfossi, Laura |
| Author affiliation(s) | University of Turin |
| Primary contact | Email: laura dot anfossi at unito dot it |
| Year published | 2020 |
| Volume and issue | 12(4) |
| Article # | 265 |
| DOI | 10.3390/toxins12040265 |
| ISSN | 2072-6651 |
| Distribution license | Creative Commons Attribution 4.0 International |
| Website | https://www.mdpi.com/2072-6651/12/4/265/htm |
| Download | https://www.mdpi.com/2072-6651/12/4/265/pdf (PDF) |
|
|
This article should be considered a work in progress and incomplete. Consider this article incomplete until this notice is removed. |
Abstract
The diffusion of the legalization of cannabis for recreational, medicinal, and nutraceutical uses requires the development of adequate analytical methods to assure the safety and security of such products. In particular, aflatoxins are considered to pose a major risk for the health of cannabis consumers. Among analytical methods that allow for adequate monitoring of food safety, immunoassays play a major role thanks to their cost-effectiveness, high-throughput capacity, simplicity, and limited requirement for equipment and skilled operators. Therefore, a rapid and sensitive enzyme immunoassay has been adapted to measure the most hazardous aflatoxin B1 in cannabis products. The assay was acceptably accurate (recovery rate: 78–136%), reproducible (intra- and inter-assay means coefficients of variation 11.8% and 13.8%, respectively), and sensitive (limit of detection and range of quantification: 0.35 ng mL−1 and 0.4–2 ng mL−1, respectively corresponding to 7 ng g−1 and 8–40 ng g−1 in the plant), while providing results which agreed with a high-performance liquid chromatography–tandem mass spectrometry (HPLC-MS/MS) method for the direct analysis of aflatoxin B1 in cannabis inflorescence and leaves. In addition, the carcinogenic aflatoxin B1 was detected in 50% of the cannabis products analyzed (14 samples collected from small retails) at levels exceeding those admitted by the European Union in commodities intended for direct human consumption, thus envisaging the need for effective surveillance of aflatoxin contamination in legal cannabis.
Keywords: mycotoxins, food safety, medicinal herbs, competitive immunoassay
Introduction
Cannabis sativa is a plant of the Cannabaceae family and is well-known for its content of biologically active chemical compounds, among which are the major compounds Δ9-tetrahydrocannabinol (Δ9-THC) and cannabidiol (CBD). The flowering or fruiting tops of the Cannabis plant have been controlled in the United States under the Controlled Substances Act since 1970 under the drug class “Marihuana.”[1]
Cannabis products can be used for medicinal purposes (whether using the psychoactive constituent THC or the non-psychoactive constituent CBD, generally referred to as "medical cannabis"), in manufacturing ("industrial hemp"), and for non-medical intoxication ("recreational or psychoactive cannabis").[2] The number of active constituents found in cannabis and the variety of their effects have also suggested cannabis' potential use as a dietary supplement and nutraceutical.[1][3] According to the World Health Organization (WHO), recreational cannabis is the most widely used illicit drug and the most largely cultivated and trafficked worldwide.[4]
The therapeutic application of cannabis is increasing around the world.[5] For example, a medicine based on cannabis extract has been approved by the European Medicines Agency.[6] THC can be medically administered as capsules, mouth spray, or as flowers for making tea. And the U.S. Food and Drug Administration (FDA) has approved one cannabis-derived and three cannabis-related drug products.[7]
The cultivation and supply of cannabis for industrial use has been legal in the European Union since 2013, provided the cannabis' THC content does not exceed 0.2%.[8] In 2018, the U.S. legalized the production and marketing of hemp, provided that its THC content is below 0.3% on a dry weight basis.[1]
As cannabis increasingly becomes legalized for recreational purposes, dietary supplements, and various medical applications, growth of the global legal market of such products looks favorable in the coming years. However, the toxicity of common cannabis contaminants to humans is largely unknown. Due to the ambiguity between legal and illicit production and supply of cannabis products, there is a significant lacking in the literature regarding the prevalence of cannabis contaminants and of their harmfulness to humans. Contemporarily, the expanded use of cannabis products demands further research in this area, especially for therapeutic uses.[9]
Several classes of contaminants can be present in cannabis, including heavy metals, which are able to bioaccumulate in Cannabis plants[10]; pesticides, (including illegal pesticides; given how long cannabis has been illegal, pesticide guidelines or maximal limits for pesticide residues have not been set for this substrate); microbiological contaminants; and toxins from microbial overloads, such as ochratoxins and aflatoxins.[11][12]
McKernan et al. showed that toxigenic fungi grow on cannabis (especially those producing ochratoxin and aflatoxin) and highlighted the need to investigate the presence of the corresponding mycotoxins in these kinds of samples.[13] Among mycotoxins that can affect cannabis, aflatoxins (AFs) are of utmost concern because of their toxicity and their widespread distribution. AFs are carcinogens, genotoxic, and immunosuppressive agents.[14] In particular, aflatoxin B1 (AFB1) is the most recurrent and carcinogenic of the aflatoxins, and it is well documented to be a causative agent of hepatocellular carcinoma as well as growth suppression, immune system modulation, and malnutrition.[15][16] AFB1 is produced by fungi of the Aspergillus genus, namely Aspergillus flavus and Aspergillus parasiticus.
A. flavus is ubiquitously found in soil and contaminates a wide range of the world’s crops. After establishing the plant as a host, the fungus produces aflatoxins, including AFB1. Fungal growth can occur on crops at any point in the pre- or post-harvest stage. Additionally, high temperatures and humidity favor fungal growth, so carelessness of storage conditions favors a large amount of AFB1 contamination occurring during storage.[17]
The lack of regulations and the prevailing illegal production, storage, and consumption of cannabis have meant a general unavailability of controls on its safety, including the absence of methods to monitor contamination. In this work, a rapid and sensitive enzyme immunoassay for measuring AFB1—primarily developed to monitor the presence of the toxin in eggs[18]—was adapted for detecting AFB1 in cannabis products. Although several accurate and sensitive immunoenzymatic kits for AFB1 detection are available on the market, the indiscriminate use of immunoassay kits originally developed and validated for application in specific matrices (to monitor AFB1 in very different materials) should be carefully evaluated. Therefore, samples of cannabis derivatives (inflorescence and leaves) legally sold under the requirement of THC content lower than 0.2% were collected in small retail outlets in Torino (Italy). The enzyme immunoassay was modified in order to comply with the effect of the herbaceous matrix and the modified assay was in-house validated. A chromatographic tandem mass spectrometry method was also developed to confirm accuracy of the enzyme immunoassay. Finally, the sensitive enzyme immunoassay was used to measure AFB1 contamination in 14 samples of cannabis products.
Results
Enzyme immunoassay adaptation to AFB1 detection in cannabis products
Extraction of aflatoxin B1 from cannabis leaves and seeds was carried out by partitioning in 80% methanol, as previously reported by other researchers for AFB1 extraction from several kinds of medical plants.[19][20]
The enzyme immunoassay used in this work was initially developed for measuring aflatoxins in eggs[18] and consisted of a direct competitive immunoassay, in which a polyclonal antibody raised against aflatoxin M1 linked to bovine serum albumin (BSA) (antiM1-pAb) was adsorbed onto the polystyrene of microplate wells. The target compound (AFB1) and the enzyme probe (AFB1 linked to horse radish peroxidase, AFB1-HRP) competed for binding to the anchored antibody. After removing unbound fractions by washing the plate, the signal generated by the enzyme was developed and measured. The time required to complete the analysis was 30 minutes. In previous work, we also produced a second polyclonal antibody using AFB1 linked to BSA as the immunogen (antiB1-pAb). The antiB1-pAb showed higher selectivity towards AFB1 compared to the antiM1-pAb and was used in this work. Therefore, optimal AFB1-HRP and antiB1-pAb concentrations were defined ex-novo through the checkerboard titration approach. Other assay parameters were also re-evaluated. In particular, AFB1-HRP and antiB1-pAb concentrations and time of reactions were decided upon, providing a signal of the blank of approximately 1.5 UA and an IC50 of the calibration curve below 1 ng mL−1. Other parameters were defined based on minimizing matrix effect. Hence, extracts were fortified with known concentrations of AFB1 and the relative matrix effect (ME%) was calculated as follows:
ME% = (AFB1 measured in the fortified extract − AFB1 measured in the non-fortified extract) / AFB1 added × 100[21]
As the scope of the re-optimization of the enzyme immunoassay was intended for coping with new interference in AFB1 quantification due to the specific composition of the cannabis matrix, recovery was measured by fortifying the extract, which included potential interfering substances deriving from the sample.
A modification of the pristine protocol was considered for statistically significant improvement of the obtained ME% rate.
Two samples (representative of leaves and inflorescence) collected in a small local retail outlet were extracted and, using the methanolic extracts fortified with AFB1, the following parameters were studied: (1) dilution of the methanolic extract with water; (2) volume of the diluted extract to be added to the reaction well; (3) time for the immuno- and enzymatic reactions; (4) nature of the buffer for AFB1-HRP dilution; and (5) composition of the washing solution.
In particular, we observed that a precipitate formed when the methanolic extracts were diluted with water; however, filtration and centrifugation to remove the particulate matter caused a dramatic loss of the toxin, measured by recovery values below 50%. Then, the raw suspension was diluted 1 + 1 and added directly to the wells. Higher dilution rate (1 + 3) decreased the sensitivity of the assay (because of sample dilution) without increasing recovery rates, while using the undiluted extract produced a strong matrix effect evidenced by a large overestimation of the AFB1. For avoiding excessive matrix interference, the sample volume was reduced to one half (further reducing sample volume was ineffective for increasing recovery and halved the sensitivity). The pH of the buffer used for the immunoreaction and of the washing solutions was also modified in order to obtain satisfying recovery rates. Specifically, lowering the pH of both solutions to 5.0 allowed us to suppress most of the matrix interference. On the contrary, modification of composition (salts and additives) of buffers did not allow us to significantly improve recovery rates (see Supplementary materials, Figure S1). Finally, the time of reactions was defined to limit the overall time required for completing the analysis while providing a signal of the blank that was measured with acceptable precision (>1 UA). The total time for the analysis was 40 minutes, which is quite low for microplate-based immunoassays and acceptable for the intended use as a first-level screening analysis.
The experimental conditions considered in the study and the protocol optimized for AFB1 detection in cannabis are shown in Table 1. Several parameters of the pristine protocol needed to be modified to achieve acceptable recovery rates in the detection of AFB1 in cannabis products instead of in egg yolk. This finding pointed out that the use of commercial kits originally intended for specific applications to different commodities without modifications can lead to inaccuracy and should be discouraged.
| |||||||||||||||||||||
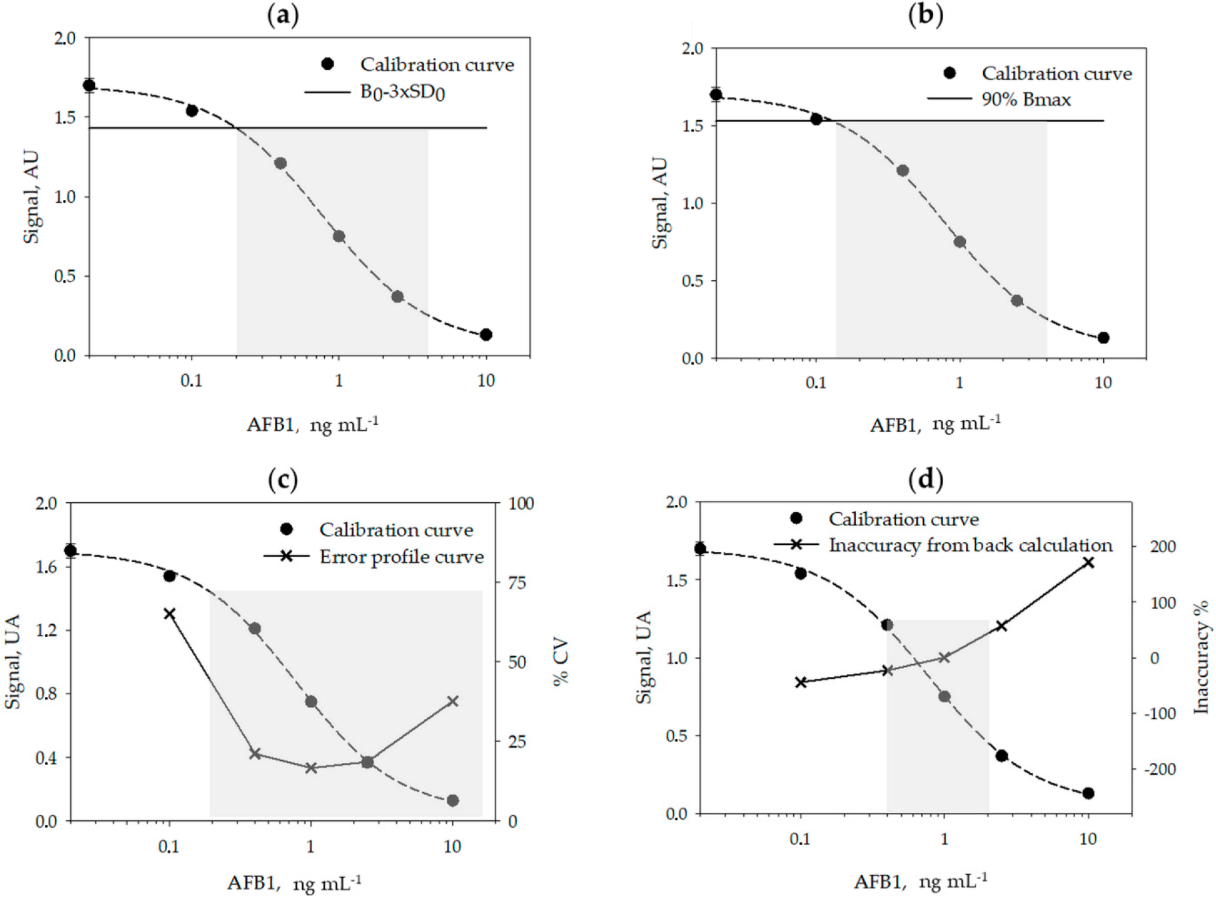
A typical calibration curve for measuring AFB1 obtained in the optimized conditions is shown in Figure 1.
|
Analytical figures of merits of the enzyme immunoassay
Using six calibration curves, generated on different days, and by using six calibrators measured in duplicate on each day, we studied the reproducibility of the calibration (Table 2) and calculated the limit of detection (LOD) and the range of quantification (ROQ) of the assay (Table 3 and Figure 1, above). Signals recorded on each day were normalized by the signal of the calibrator containing no AFB1 (B0). The LOQ and ROQ were estimated according to four methods, variously applied to competitive immunoassays: the signal-to-noise method[21][22], the IC10/20–80 method[23][24][25][26], the error profile method[27], and the back-calculation method.[28]
| ||||||||||||
| ||||||||||||||||||||||||||||||||||||
References
- ↑ 1.0 1.1 1.2 U.S. Food and Drug Administration. "FDA Regulation of Cannabis and Cannabis-Derived Products, Including Cannabidiol (CBD)". U.S. Food and Drug Administration. https://www.fda.gov/news-events/public-health-focus/fda-regulation-cannabis-and-cannabis-derived-products-including-cannabidiol-cbd. Retrieved 10 July 2019.
- ↑ Mead, A. (2019). "Legal and Regulatory Issues Governing Cannabis and Cannabis-Derived Products in the United States". Frontiers in Plant Science 10: 697. doi:10.3389/fpls.2019.00697. PMC PMC6590107. PMID 31263468. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6590107.
- ↑ Hartsel, J.A.; Eades, J.; Hickory, B.; Makriyannis, A. (2016). "Chapter 53: Cannabis sativa and Hemp". In Gupta, R.C.. Nutraceuticals: Efficacy, Safety and Toxicity. Academic Press. pp. 735–754. ISBN 9780128021477.
- ↑ World Health Organization. "Cannabis". Management of substance abuse. World Health Organization. https://www.who.int/substance_abuse/facts/cannabis/en/. Retrieved 20 April 2020.
- ↑ Bridgeman, M.B.; Abazia, D.T. (2017). "Medicinal Cannabis: History, Pharmacology, And Implications for the Acute Care Setting". P & T 42 (3): 180–88. PMC PMC5312634. PMID 28250701. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5312634.
- ↑ European Monitoring Centre for Drugs and Drug Addiction (December 2018). "Medical use of cannabis and cannabinoids: Questions and answers for policymaking". EMCDDA. doi:0.2810/979004. https://www.emcdda.europa.eu/publications/rapid-communications/medical-use-of-cannabis-and-cannabinoids-questions-and-answers-for-policymaking_en. Retrieved 04 November 2019.
- ↑ Corroon, J.; Kight, R. (2018). "Regulatory Status of Cannabidiol in the United States: A Perspective". Cannabis and Cannabinoid Research 3 (1): 190-194. doi:10.1089/can.2018.0030. PMC PMC6154432. PMID 30283822. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6154432.
- ↑ "Regulation (EU) No 1307/2013 of the European Parliament and of the Council". Official Journal of the European Union. 20 December 2013. pp. 608–70. https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2013:347:0608:0670:EN:PDF.
- ↑ Dryburgh, L.M.; Bolan, N.S.; Grof, C.P.L. et al. (2018). "Cannabis Contaminants: Sources, Distribution, Human Toxicity and Pharmacologic Effects". British Journal of Clinical Pharmacology 84 (11): 2468-2476. doi:10.1111/bcp.13695. PMC PMC6177718. PMID 29953631. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6177718.
- ↑ Zerihun, A. Chandravanshi, B.S.; Debebe, A. et al. (2015). "Levels of Selected Metals in Leaves of Cannabis Sativa L. Cultivated in Ethiopia". SpringerPlus 4: 359. doi:10.1186/s40064-015-1145-x. PMC PMC4503701. PMID 26191486. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4503701.
- ↑ Llewellyn, G.C.; O'Rear, C.E. (1977). "Examination of Fungal Growth and Aflatoxin Production on Marihuana". Mycopathologia 62 (2): 109–12. doi:10.1007/BF01259400. PMID 414138.
- ↑ Wilcox, J.; Pazdanska, M.; Milligan, C. (2020). "Analysis of Aflatoxins and Ochratoxin A in Cannabis and Cannabis Products by LC-Fluorescence Detection Using Cleanup With Either Multiantibody Immunoaffinity Columns or an Automated System With In-Line Reusable Immunoaffinity Cartridges". Journal of AOAC International 103 (2): 494–503. doi:10.5740/jaoacint.19-0176. PMID 31558181.
- ↑ McKernan, K.; Spangler, J.; Zhang, L. et al. (2015). "Cannabis Microbiome Sequencing Reveals Several Mycotoxic Fungi Native to Dispensary Grade Cannabis Flowers". F1000Research 4: 1422. doi:10.12688/f1000research.7507.2. PMC PMC4897766. PMID 27303623. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4897766.
- ↑ European Food Safety Authority. "Aflatoxins in food". https://www.efsa.europa.eu/en/topics/topic/aflatoxins-food. Retrieved 10 July 2019.
- ↑ Marchese, S.; Polo, A.; Ariano, A. et al. (2018). "Aflatoxin B1 and M1: Biological Properties and Their Involvement in Cancer Development". Toxins 10 (6): 214. doi:10.3390/toxins10060214. PMC PMC6024316. PMID 29794965. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6024316.
- ↑ International Agency for Research on Cancer (2002). Some Traditional Herbal Medicines, Some Mycotoxins, Naphthalene and Styrene. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. 82. pp. 171–274. doi:10.3390/toxins10060214. PMC PMC6024316. PMID 29794965.
- ↑ Rushing, B.R.; Selim, M.I. (2019). "Aflatoxin B1: A Review on Metabolism, Toxicity, Occurrence in Food, Occupational Exposure, and Detoxification Methods". Food and Chemical Toxicology 124: 81–100. doi:10.1016/j.fct.2018.11.047. PMID 30468841.
- ↑ 18.0 18.1 18.2 Anfossi, L.; Di Nardo, F.; Giovannoli, C. et al. (2015). "Enzyme immunoassay for monitoring aflatoxins in eggs". Food Control 57: 115–21. doi:10.1016/j.foodcont.2015.04.013.
- ↑ Ventura, M.; Gómez, A.; Anaya, I. et al. (2004). "Determination of Aflatoxins B1, G1, B2 and G2 in Medicinal Herbs by Liquid Chromatography-Tandem Mass Spectrometry". Journal of Chromatography A 1048 (1): 25–9. doi:10.1016/j.chroma.2004.07.033. PMID 15453415.
- ↑ Arranz, I.; Sizoo, E.; van Egmond, H. et al. (2006). "Determination of Aflatoxin B1 in Medical Herbs: Interlaboratory Study". Journal of AOAC International 89 (3): 595-605. doi:10.1093/jaoac/89.3.595. PMID 16792057.
- ↑ 21.0 21.1 21.2 21.3 21.4 Zhang, Z.; Dong, S.; Ge, D. et al. (2018). "An Ultrasensitive Competitive Immunosensor Using Silica Nanoparticles as an Enzyme Carrier for Simultaneous Impedimetric Detection of Tetrabromobisphenol A bis(2-hydroxyethyl) Ether and Tetrabromobisphenol A Mono(hydroxyethyl) Ether". Biosensors and Bioelectronics 105: 77–80. doi:10.1016/j.bios.2018.01.029. PMID 29355782.
- ↑ 22.0 22.1 22.2 22.3 Reimer, G.J.; Gee, S.J.; Hammock, B.D. (1998). "Comparison of a Time-Resolved Fluorescence Immunoassay and an Enzyme-Linked Immunosorbent Assay for the Analysis of Atrazine in Water". Journal of Agricultural and Food Chemistry 46 (8): 3353–58. doi:10.1021/jf970965a.
- ↑ 23.0 23.1 23.2 23.3 Sasaki, D.; Mitchell, R.A. (2001). "How to Obtain Reproducible Quantitative ELISA Results" (PDF). Oxford Biomedical Research, Inc. https://www.oxfordbiomed.com/sites/default/files/2017-02/How%20to%20Obtain%20Reproducible%20Quantitative%20ELISA%20results.pdf.
- ↑ 24.0 24.1 24.2 24.3 Saeed, A.F.U.H.; Ling, S.; Yuan, J. et al. (2017). "The Preparation and Identification of a Monoclonal Antibody Against Domoic Acid and Establishment of Detection by Indirect Competitive ELISA". Toxins 9 (8): 250. doi:10.3390/toxins9080250. PMC PMC5577584. PMID 28817087. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5577584.
- ↑ 25.0 25.1 25.2 25.3 Zhang, X.; Yu, X.; Wen, K. et al. (2017). "Multiplex Lateral Flow Immunoassays Based on Amorphous Carbon Nanoparticles for Detecting Three Fusarium Mycotoxins in Maize". Journal of Agricultural and Food Chemistry 65 (36): 8063-8071. doi:10.1021/acs.jafc.7b02827. PMID 28825819.
- ↑ 26.0 26.1 26.2 26.3 Zhang, Z.; Zhu, N.; Zou, Y. et al. (2018). "A Novel and Sensitive Chemiluminescence Immunoassay Based on AuNCs@pepsin@luminol for Simultaneous Detection of Tetrabromobisphenol A bis(2-hydroxyethyl) Ether and Tetrabromobisphenol A Mono(hydroxyethyl) Ether". Analytica Chimica Acta 1035: 168-174. doi:10.1016/j.aca.2018.06.039. PMID 30224136.
- ↑ 27.0 27.1 27.2 27.3 Quinn, C.P.; Semenova, V.A.; Elie, C.M. et al. (2002). "Specific, Sensitive, and Quantitative Enzyme-Linked Immunosorbent Assay for Human Immunoglobulin G Antibodies to Anthrax Toxin Protective Antigen". Emerging Infectious Diseases 8 (10): 1103-10. doi:10.3201/eid0810.020380. PMC PMC2730307. PMID 12396924. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2730307.
- ↑ 28.0 28.1 28.2 28.3 Dunn, J.; Wild, D. (2013). "Calibration Curve Fitting". In Wild, D.. The Immunoassay Handbook (3rd ed.). Elsevier Science. pp. 323–336. ISBN 9780080445267.
Notes
This presentation is faithful to the original, with only a few minor changes to presentation. Some grammar and punctuation was cleaned up to improve readability. In some cases important information was missing from the references, and that information was added. In the original article, citations 1 and 4 are duplicates; that duplication was removed for this version.