LII:Basic Physics of Nuclear Medicine/Nuclear Medicine Imaging Systems

Topics we have covered in this wikibook have included radioactivity, the interaction of gamma-rays with matter and radiation detection. The main reason for following this pathway was to bring us to the subject of this chapter: nuclear medicine imaging systems. These are devices which produce pictures of the distribution of radioactive material following administration to a patient.
The radioactivity is generally administered to the patient in the form of a radiopharmaceutical – the term radiotracer is also used. This follows some physiological pathway to accumulate for a short period of time in some part of the body. A good example is 99mTc-tin colloid which following intravenous injection accumulates mainly in the patient's liver. The substance emits gamma-rays while it is in the patient's liver and we can produce an image of its distribution using a nuclear medicine imaging system. This image can tell us whether the function of the liver is normal or abnormal or if sections of it are damaged from some form of disease.
Different radiopharmaceuticals are used to produce images from almost every region of the body:
| Part of the Body | Example Radiotracer |
|---|---|
| Brain | 99mTc-HMPAO |
| Thyroid | Na99mTcO4 |
| Lung (Ventilation) | 133Xe gas |
| Lung (Perfusion) | 99mTc-MAA |
| Liver | 99mTc-Tin (or Sulphur) Colloid |
| Spleen | 99mTc-Damaged Red Blood Cells |
| Pancreas | 75Se-Selenomethionine |
| Kidneys | 99mTc-DMSA |
Note that the form of information obtained using this imaging method is mainly related to the physiological functioning of an organ as opposed to the mainly anatomical information which is obtained using X-ray imaging systems. Nuclear medicine therefore provides a different perspective on a disease condition and generates additional information to that obtained from X-ray images. Our purpose here is to concentrate on the imaging systems used to produce the images.
Early forms of imaging system used in this field consisted of a radiation detector (a scintillation detector for example) which was scanned slowly over a region of the patient in order to measure the radiation intensity emitted from individual points within the region. One such device was called the Rectilinear Scanner. Such imaging systems have been replaced since the 1970s by more sophisticated devices which produce images much more rapidly. The most common of these modern devices is called the Gamma Camera and we will consider its construction and mode of operation below. A review of recent developments in this technology for cardiac applications can be found in Slomka et al (2009)[1].
Gamma Camera
[edit | edit source]The basic design of the most common type of gamma camera used today was developed by an American physicist, Hal Anger and is therefore sometimes called the Anger Camera. It consists of a large diameter NaI(Tl) scintillation crystal which is viewed by a large number of photomultiplier tubes.
A block diagram of the basic components of a gamma camera is shown below:

The crystal and PM Tubes are housed in a cylindrical shaped housing commonly called the camera head and a cross-sectional view of this is shown in the figure. The crystal can be between about 25 cm and 40 cm in diameter and about 1 cm thick. The diameter is dependent on the application of the device. For example a 25 cm diameter crystal might be used for a camera designed for cardiac applications while a larger 40 cm crystal would be used for producing images of the lungs. The thickness of the crystal is chosen so that it provides good detection for the 140 keV gamma-rays emitted from 99mTc – which is the most common radioisotope used today.
Scintillations produced in the crystal are detected by a large number of PM tubes which are arranged in a two-dimensional array. There are typically between 37 and 91 PM tubes in modern gamma cameras. The output voltages generated by these PM tubes are fed to a position circuit which produces four output signals called ±X and ±Y. These position signals contain information about where the scintillations were produced within the crystal. In the most basic gamma camera design they are fed to a cathode ray oscilloscope (CRO). We will describe the operation of the CRO in more detail below.
Before we do so we should note that the position signals also contain information about the intensity of each scintillation. This intensity information can be derived from the position signals by feeding them to a summation circuit (marked ∑ in the figure) which adds up the four position signals to generate a voltage pulse which represents the intensity of a scintillation. This voltage pulse is commonly called the Z-pulse which, following pulse height analysis, (PHA) is fed as the unblank pulse to the CRO.
So we end up with four position signals and an unblank pulse sent to the CRO. Let us briefly review the operation of a CRO before we continue. The core of a CRO consists of an evacuated tube with an electron gun at one end and a phosphor-coated screen at the other end. The electron gun generates an electron beam which is directed at the screen and the screen emits light at those points struck by the electron beam. The position of the electron beam can be controlled by vertical and horizontal deflection plates and with the appropriate voltages fed to these plates the electron beam can be positioned at any point on the screen. The normal mode of operation of an oscilloscope is for the electron beam to remain switched on. In the case of the gamma camera the electron beam of the CRO is normally switched off – it is said to be blanked.
When an unblank pulse is generated by the PHA circuit the electron beam of the CRO is switched on for a brief period of time so as to display a flash of light on the screen. In other words the voltage pulse from the PHA circuit is used to unblank the electron beam of the CRO.
So where does this flash of light occur on the screen of the CRO? The position of the flash of light is dictated by the ±X and ±Y signals generated by the position circuit. These signals as you might have guessed are fed to the deflection plates of the CRO so as to cause the unblanked electron beam to strike the screen at a point related to where the scintillation was originally produced in the NaI(Tl) crystal. Simple!
The gamma camera can therefore be considered to be a sophisticated arrangement of electronic circuits used to translate the position of a flash of light in a scintillation crystal to a flash of light at a related point on the screen of an oscilloscope. In addition the use of a pulse height analyser in the circuitry allows us to translate the scintillations related only to photoelectric events in the crystal by rejecting all voltage pulses except those occurring within the photopeak of the gamma-ray energy spectrum.
Let us summarise where we have got to before we proceed. A radiopharmaceutical is administered to the patient and it accumulates in the organ of interest. Gamma-rays are emitted in all directions from the organ and those heading in the direction of the gamma camera enter the crystal and produce scintillations (note that there is a device in front of the crystal called a collimator which we will discuss later). The scintillations are detected by an array of PM tubes whose outputs are fed to a position circuit which generates four voltage pulses related to the position of a scintillation within the crystal. These voltage pulses are fed to the deflection circuitry of the CRO. They are also fed to a summation circuit whose output (the Z-pulse) is fed to the PHA and the output of the PHA is used to switch on (that is, unblank) the electron beam of the CRO. A flash of light appears on the screen of the CRO at a point related to where the scintillation occurred within the NaI(Tl) crystal. An image of the distribution of the radiopharmaceutical within the organ is therefore formed on the screen of the CRO when the gamma-rays emitted from the organ are detected by the crystal.
What we have described above is the operation of a fairly traditional gamma camera. Modern designs are a good deal more complex but the basic design has remained much the same as has been described. One area where major design improvements have occurred is the area of image formation and display. The most basic approach to image formation is to photograph the screen of the CRO over a period of time to allow integration of the light flashes to form an image on photographic film. A stage up from this is to use a storage oscilloscope which allows each flash of light to remain on the screen for a reasonable period of time.
The most modern approach is to feed the position and energy signals into the memory circuitry of a computer for storage. The memory contents can therefore be displayed on a computer monitor and can also be manipulated (that is processed) in many ways. For example various colours can be used to represent different concentrations of a radiopharmaceutical within an organ.
The use of digital image processing is now widespread in nuclear medicine in that it can be used to rapidly and conveniently control image acquisition and display as well as to analyse an image or sequences of images, to annotate images with the patient's name and examination details, to store the images for subsequent retrieval and to communicate the image data to other computers over a network.
The essential elements of a modern gamma camera are shown in the next figure. Gamma rays emitted by the patient pass through the collimator and are detected within the camera head, which generates data related to the location of scintillations in the crystal as well as to the energy of the gamma rays. This data is then processed on-the-fly by electronic hardware which corrects for technical factors such as spatial linearity, PM tube drift and energy response so as to produce an imaging system with a spatially-uniform sensitivity and distortion-free performance.
A multichannel analyzer (MCA) is used to display the energy spectrum of gamma rays which interact inside the crystal. Since these gamma rays originate from within the patient, some of them will have an energy lower than the photopeak as a result of being scattered as they travel through the patient's tissues – and by other components such as the patient table and structures of the imaging system. Some of these scattering events may involve just glancing interactions with free electrons, so that the gamma rays lose only a small amount of energy. These gamma rays may have an energy just below that of the photopeak so that their spectrum merges with the photopeak. The photopeak for a gamma camera imaging a patient therefore contains information from spatially-correlated, unattenuated gamma rays (which is the information we want) and from spatially-uncorrelated, scattered gamma rays. The scattered gamma rays act like a variable background within the true photopeak data and the effect is that of a background haze in gamma camera images.

While scatter may not be a significant problem in planar scintigraphy, it has a strong bearing on the fidelity of quantitative information derived from gamma camera images and is a vital consideration for accurate image reconstruction in emission tomography. It is the unattenuated gamma rays (also called the primary radiation) that contain the desired information, because of their direct dependence on radioactivity.
The scatter situation is illustrated in more detail in the figure below, which shows estimates of the primary and scatter spectra for 99mTc in patient imaging conditions. Such spectral estimates can be generated using Monte Carlo methods. It is seen in the figure that the energy of the scattered radiation forms a broad band, similar to the Compton Smear described previously, which merges into and contributes substantially to the detected photopeak. The detected photopeak is therefore an overestimate of the primary radiation. The extent of this overestimate is likely to be dependent on the specific imaging situation because of the different thicknesses of tissues involved. It is clear however that the scatter contribution within the detected photopeak needs to be accounted for if an accurate measure of radioactivity is required.

One method of compensating for the scatter contribution is illustrated in the figure below and involves using data from a lower energy window as an estimate for subtraction from the photopeak, i.e.
where k is a scaling factor to account for the extent of the scatter contribution. This approach to scatter compensation is referred to as the Dual-Energy Window (DEW) method. It can be implemented in practice by acquiring two images, one for each energy window, and subtracting a fraction (k) of the scatter image from the photopeak image.
For the spectrum shown above, it can be seen that the scaling factor, k, is about 0.5, but it should be appreciated that its exact value is dependent on the scattering conditions. Gamma cameras which use the DEW method therefore generally provide the capability of adjusting k for different imaging situations. Some systems use a narrower scatter window than that illustrated, e.g. 114-126 keV, with a consequent increase in k to about 1.0, for instance.
A host of other methods of scatter compensation have also been developed. These include more complex forms of energy analysis such as the Dual-Photopeak and the Triple-Energy Window techniques, as well as approaches based on deconvolution and models of photon attenuation. An excellent review of these developments is provided in Zaidi & Koral (2004).

Some photographs of gamma cameras and related devices are shown below:
We will continue with our description of the gamma camera by considering the construction and purpose of the collimator.
Collimation
[edit | edit source]The collimator is a device which is attached to the front of the gamma camera head. It functions something like a lens used in a photographic camera but this analogy is not quite correct because it is rather difficult to focus gamma-rays. Nevertheless in its simplest form it is used to block out all gamma rays which are heading towards the crystal except those which are travelling at right angles to the plane of the crystal:

The figure illustrates a magnified view of a parallel-hole collimator attached to a crystal. The collimator simply consists of a large number of small holes drilled in a lead plate. Notice that gamma-rays entering at an angle to the crystal get absorbed by the lead and that only those entering along the direction of the holes get through to cause scintillations in the crystal. If the collimator was not in place these obliquely incident gamma-rays would blur the images produced by the gamma camera. In other words the images would not be very clear.
Most gamma cameras have a number of collimators which can be fitted depending on the examination. The basic design of these collimators is the same except that they vary in terms of the diameter of each hole, the depth of each hole and the thickness of lead between each hole (commonly called the septum thickness). The choice of a specific collimator is dependent on the amount of radiation absorption that occurs (which influences the sensitivity of the gamma camera), and the clarity of images (that is the spatial resolution) it produces. Unfortunately these two factors are inversely related in that the use of a collimator which produces images of good spatial resolution generally implies that the instrument is not very sensitive to radiation.
Other collimator designs beside the parallel hole type are also in use. For example a diverging hole collimator produces a minified image and converging hole and pin-hole collimators produce a magnified image. The pin-hole collimator is illustrated in the following figure:

It is typically a cone-shaped device with its walls made from lead. A cross-section through this cone is shown in the figure. It operates in a similar fashion to a pin-hole photographic camera and produces an inverted image of an object – an arrow is used in the figure to illustrate this inversion. This type of collimator has been found useful for imaging small objects such as the thyroid gland.
Example Images
[edit | edit source]A representative selection of nuclear medicine images is shown below:
Emission Tomography
[edit | edit source]The form of imaging which we have been describing is called Planar Imaging. It produces a two-dimensional image of a three-dimensional object. As a result images contain no depth information and some details can be superimposed on top of each other and obscured or partially obscured as a result. Note that this is also a feature of conventional X-ray imaging.
The usual way of trying to overcome this limitation is to take at least two views of the patient, one from the front and one from the side for example. So in chest radiography a posterio-anterior (PA) and a lateral view can be taken. And in a nuclear medicine liver scan an antero-posterior (AP) and lateral scan are acquired.
This limitation of planar X-ray imaging was overcome by the development of the CAT Scanner about 1970 or thereabouts. CAT stands for Computerized Axial Tomography or Computer Assisted Tomography and today the term is often shortened to Computed Tomography or CT scanning (the term tomography comes from the Greek word tomos meaning slice). Irrespective of its exact name the technique allows images of slices through the body to be produced using a computer. It does this in essence by taking X-ray images at a number of angles around the patient. These slice images show the third dimension which is missing from planar images and thus eliminate the problem of superimposed details. Furthermore images of a number of successive slices through a region of the patient can be stacked on top of each other using the computer to produce a three-dimensional image. Clearly CT scanning is a very powerful imaging technique relative to planar imaging.
The equivalent nuclear medicine imaging technique is called Emission Computed Tomography. We will consider two implementations of this technique below.
Single Photon Emission Computed Tomography (SPECT)
[edit | edit source]- This SPECT technique uses a gamma camera to record images at a series of angles around the patient. These images are then subjected to a form of digital image processing called Image Reconstruction in order to compute images of slices through the patient.
- The Back Projection reconstruction process is illustrated below. Let us assume for simplicity that the slice through the patient actually consists of a 2x2 voxel array with the radioactivity in each voxel given by A1...A4:

- The first projection, P1, is imaged from the right and the second projection, P2, from the right oblique and so on. The back projection process involves firstly adding the projections to each other as shown below:

- and then normalising the summed (or superimposed) projections to generate an estimate of the radioactivity in each voxel. Since this process can generate streaking artefacts in reconstructed images, the projections are generally filtered prior to back projection, as described in a later chapter, with the overall process referred to as Filtered Back Projection (FBP):

- An alternative image reconstruction technique is called Iterative Reconstruction, a successive approximation technique. The Maximum-Likelihood Expectation-Maximisation (ML-EM) algorithm is widely applied where a division process is used to compare the actual and estimated projections, as shown below:

- One cycle of data through this processing chain is referred to as one iteration. Sixteen or more iterations can be required in order to generate an adequate reconstruction and, as a result, computation times can be rather long. The Ordered-Subsets Expectation-Maximisation (OS-EM) algorithm can be used to substantially reduce the computation time by utilising a limited number of projections (called subsets) in a sequential fashion within the iterative process. Noise generated during the reconstruction process can be reduced, for example, using a Gaussian filter built into the reconstruction calculations or applied as a post-filter:

- Images generated using different iterations, subsets and filtration settings can be found in an online book.
- A comparison of these image reconstruction techniques is shown below for a slice through a ventilation scan of a patient's lungs:

- The gamma camera is typiclly rotated around the patient in order to acquire the images. Modern gamma cameras which are designed specifically for SPECT scanning can consist of two camera heads mounted parallel to each other with the patient in between. The time required to produce images is therefore reduced by a factor of about two. In addition some SPECT gamma cameras designed for brain scanning have three camera heads mounted in a triangular arrangement.
- A wide variety of strategies can be used for the acquisition and processing of SPECT images.
Positron Emission Tomography (PET)
[edit | edit source]- You will remember from chapter 2 that positrons can be emitted from radioactive nuclei which have too many neutrons for stability. You will also remember that positrons do not last for very long in matter since they will quickly encounter an electron and a process called annihilation results. In the process the positron and electron vanish and their energy is converted into two gamma-rays which are emitted at roughly 180o degrees to each other. The emission is often referred to as two back-to-back gamma-rays and they each have a discrete energy of 0.51 MeV.
- So if we administer a positron-emitting radiopharmaceutical to a patient an emitted positrons can annihilate with a nearby electron and two gamma-rays will be emitted in opposite directions. These gamma-rays can be detected using a ring of radiation detectors encircling the patient and tomographic images can be generated using a computer system. The detectors are typically specialised scintillation devices which are optimised for detection of the 0.51 MeV gamma-rays. This ring of detectors, associated apparatus and computer system are called a PET Scanner:

- The locations of positron decays within the patient are highlighted by the solid circles in the above diagram. In addition only a few detectors are shown in the diagram for reasons of clarity. Each detector around the ring is operated in coincidence with a bank of opposing detectors and the annihilation gamma-rays thus detected are used to build up a single profile.
- It has also been found that gamma cameras fitted with thick crystals and special collimators can be used for PET scanning.
- The radioisotopes used for PET scanning include 11C, 13N, 15O and 18F. These isotopes are usually produced using an instrument called a cyclotron. In addition these isotopes have relatively short half lives. PET scanning therefore needs a cyclotron and associated radiopharmaceutical production facilities located close by. We will consider cyclotrons in the next chapter of this wikibook.
- Standardized Uptake Value (SUV) is a semi-quantitative index used in PET to express the uptake of a radiopharmaceutical in a region of interest of a patient's scan. Its typically calculated as the ratio of the radioactivity in the region to the injected dose, corrected for body weight. It should be noted that the SUV is influenced by several major sources of variability and it therefore should not be used as a quantitative measure.
- A number of photographs of a PET scanner are shown below:
 |
 |
 |
 |
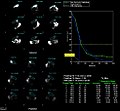
Images reconstructed using different settings of subsets and iterations for iterative reconstruction[2] are shown below:

References
[edit | edit source]- ↑ Slomka PJ, Patton JA, Berman DS & Germano G, 2009. Advances in technical aspects of myocardial perfusion SPECT imaging. Journal of Nuclear Cardiology, 16(2), 255–76.
- ↑ Maher KP, 2016. Iterations, Subsets & Gaussian Filtration, 3rd Edition (Bookemon.com]
External links
[edit | edit source]- Centre for Positron Emission Tomography at the Austin & Repatriation Medical Centre, Melbourne with sections on what PET is, current facilities, projects & research and a PET image library
Notes
This article is a direct transclusion of the Wikibooks article and therefore may not meet the same editing standards as LIMSwiki.