Journal:Quality control of cannabis inflorescence and oil products: Response factors for the cost-efficient determination of ten cannabinoids by HPLC
| Full article title | Quality control of cannabis inflorescence and oil products: Response factors for the cost-efficient determination of ten cannabinoids by HPLCn |
|---|---|
| Journal | Talanta Open |
| Author(s) | Hall, Damian R.; Sinclair, Justin S.; Bhuyan, Deep J.; Khoo, Cheang; Li, Chun G.; Sarris, Jerome; Low, Mitchell |
| Author affiliation(s) | NICM Health Research Institute, Wentworth Institute, Florey Institute of Neuroscience and Mental Health |
| Primary contact | Email: Mitchell dot Low at westernsydney dot edu dot au |
| Year published | 2022 |
| Volume and issue | 5 |
| Article # | 100112 |
| DOI | 10.1016/j.talo.2022.100112 |
| ISSN | 2666-8319 |
| Distribution license | Creative Commons Attribution 4.0 International |
| Website | https://www.sciencedirect.com/science/article/pii/S2666831922000315 |
| Download | https://www.sciencedirect.com/science/article/pii/S2666831922000315/pdfft (PDF) |
|
|
This article should be considered a work in progress and incomplete. Consider this article incomplete until this notice is removed. |
|
|
This article contains rendered mathematical formulae. You may require the TeX All the Things plugin for Chrome or the Native MathML add-on and fonts for Firefox if they don't render properly for you. |
Abstract
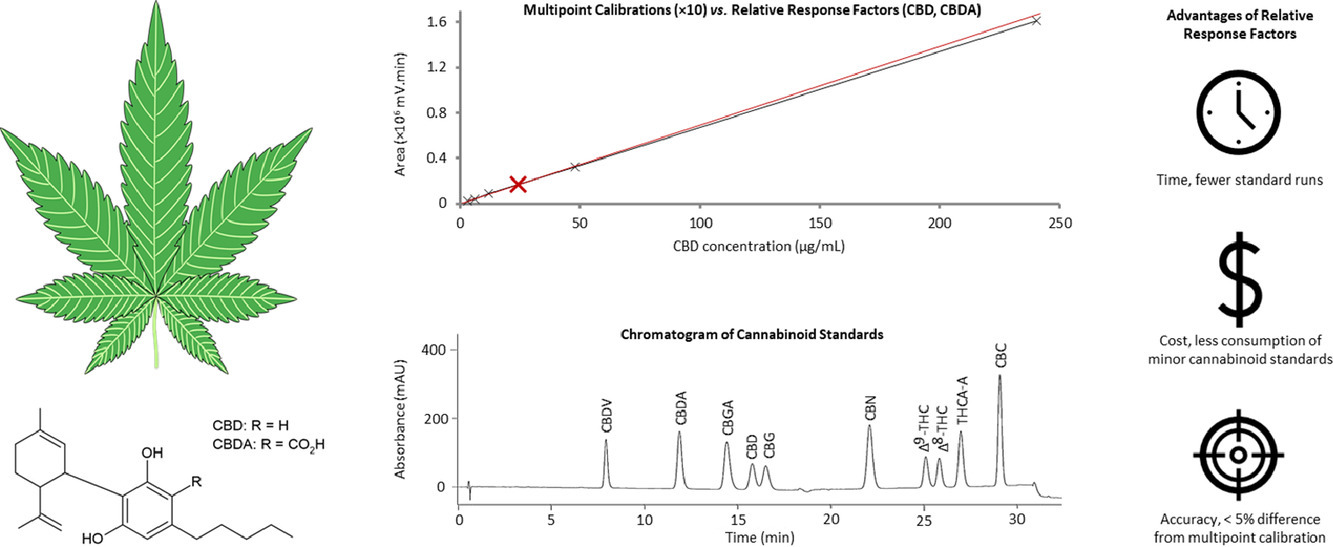
The quality control of medicinal cannabis should include quantification of as many cannabinoids as practicable in a routine analytical laboratory, to accurately reflect the quality of the product. However, the cost and availability of some cannabinoid standards is an impediment to their routine use. This work seeks to overcome this obstacle by analyzing samples using relative retention times (RRT) and relative response factors (RRF), relative to cannabidiol (CBD) and cannabidiolic acid (CBDA) reference standards which are readily available. A high-performance liquid chromatography-photodiode array method was developed to quantify 10 cannabinoids—tetrahydrocannabinol (Δ9-THC), delta-8-Tetrahydrocannabinol (Δ8-THC), delta-9-Tetrahydrocannabinolic acid A (THCA-A), cannabinol (CBN), cannabidiol (CBD), cannabidiolic acid (CBDA), cannabichromene (CBC), cannabidivarin (CBDV), cannabigerol (CBG), and cannabigerolic acid (CBGA)—in dried cannabis inflorescence and cannabis oil. This method was validated according to International Conference for Harmonization (ICH) guidelines.
The proposed method has detection limits ranging from 20 to 78 µg/g, which provided sufficient sensitivity for the panel of cannabinoids. Non-cannabinoid surrogate matrices were used for spike recovery studies to determine method accuracy; analyte recoveries for the inflorescence and oil ranged from 90.1 to 109.3% (inflorescence mean, 100.9%; oil mean, 99.6%). The RRT and RRF values, determined independently by three analysts, were comparable, indicating the method is robust. The validity of analysis using RRT and RRF was further confirmed by testing six inflorescence samples, as it was found that concentrations above the order of magnitude of the limit of quantitation (LoQ) agreed satisfactorily (range, 95.0 to 111.9%; mean, 100.0%) with the concentrations obtained through the conventional approach of multipoint calibration using pure standards. The proposed method is therefore suitable for the rapid and simple determination of a panel of 10 cannabinoids without having to repeatedly purchase every expensive pure standard. Accordingly, analysts in the medicinal cannabis field may explore the use of RRF and RRT for their methods and instruments.
Keywords: liquid chromatography, cannabis, cannabinoid, response factor, relative retention time
Introduction
References
Notes
This presentation is faithful to the original, with only a few minor changes to presentation. Some grammar and punctuation was cleaned up to improve readability. In some cases important information was missing from the references, and that information was added.