LII:Elements of Laboratory Technology Management
Title: Elements of Laboratory Technology Management
Author for citation: Joe Liscouski, with editorial modifications by Shawn Douglas
License for content: Creative Commons Attribution 4.0 International
Publication date: 2014
|
|
This article should be considered a work in progress and incomplete. Consider this article incomplete until this notice is removed. |
Introduction
This discussion is less about specific technologies than it is about the ability to use advanced laboratory technologies effectively. “Effectively” means that products and technologies are used successfully to address needs in your lab, and that they improve the lab’s ability to function. If they don't do that, you’ve wasted your money. Additionally, if the technology in question hasn’t been deployed according to a deliberate plan, your funded projects may not achieve everything they could. Optimally, when applied thoughtfully, the available technologies should result in the transformation of lab work from a labor-intensive effort to one that is intellectually intensive, making the most effective use of people and resources.
People come to the subject of laboratory automation from widely differing perspectives. To some it’s about robotics, to others it’s about laboratory informatics, and even others view it as simply data acquisition and analysis. It all depends on what your interests are, and more importantly what your immediate needs are.
People began working in this field in the 1940s and 1950s, with the work focused on analog electronics to improve instrumentation; this was the first phase of lab automation. Most notably were the development of scanning spectrophotometers and process chromatographs. Those who first encountered this equipment didn’t think much of it and considered it the world as it’s always been. Others who had to deal with products like the Spectronic 20[a] (a single-beam manual spectrophotometer), and use it to develop visible spectra one wavelength measurement at a time, appreciated the automation of scanning instruments.
Mercury switches and timers triggered by cams on a rotating shaft provided chromatographs with the ability to automatically take samples, actuate back flush valves, and take care of other functions without operator intervention. This left the analyst with the task of measuring peaks, developing calibration curves, and performing calculations, at least until data systems became available.
The direction of laboratory automation changed significantly when computer chips became available. In the 1960s, companies such as PerkinElmer were experimenting with the use of computer systems for data acquisition as precursors to commercial products. The availability of general-purpose computers such as the PDP-8 and PDP-12 series (along with the Lab 8e) from Digital Equipment, with other models available from other vendors, made it possible for researchers to connect their instruments to computers and carry out experiments. The development of microprocessors from Intel (4004, 8008) led to the evolution of “intelligent” laboratory equipment ranging from processor-controlled stirring hot-plates to chromatographic integrators.
As researchers learned to use these systems, their application rapidly progressed from data acquisition to interactive control of the experiments, including data storage, analysis, and reporting. Today, the product set available for laboratory applications includes data acquisition systems, laboratory information management systems (LIMS), electronic laboratory notebooks (ELNs), laboratory robotics, and specialized components to help researchers, scientists, and technicians apply modern technologies to their work.
While there is a lot of technology available, the question remains "how do you go about using it?" Not only do we need to know how to use it, but we also must do so while avoiding our own biases about how computer systems operate. Our familiarity with using computer systems in our daily lives may cause us to assume they are doing what we need them to do, without questioning how it actually gets done. “The vendor knows what they are doing” is a poor reason for not testing and evaluating control parameters to ensure they are suitable and appropriate for your work.
Moving from lab functions and requirements to practical solutions
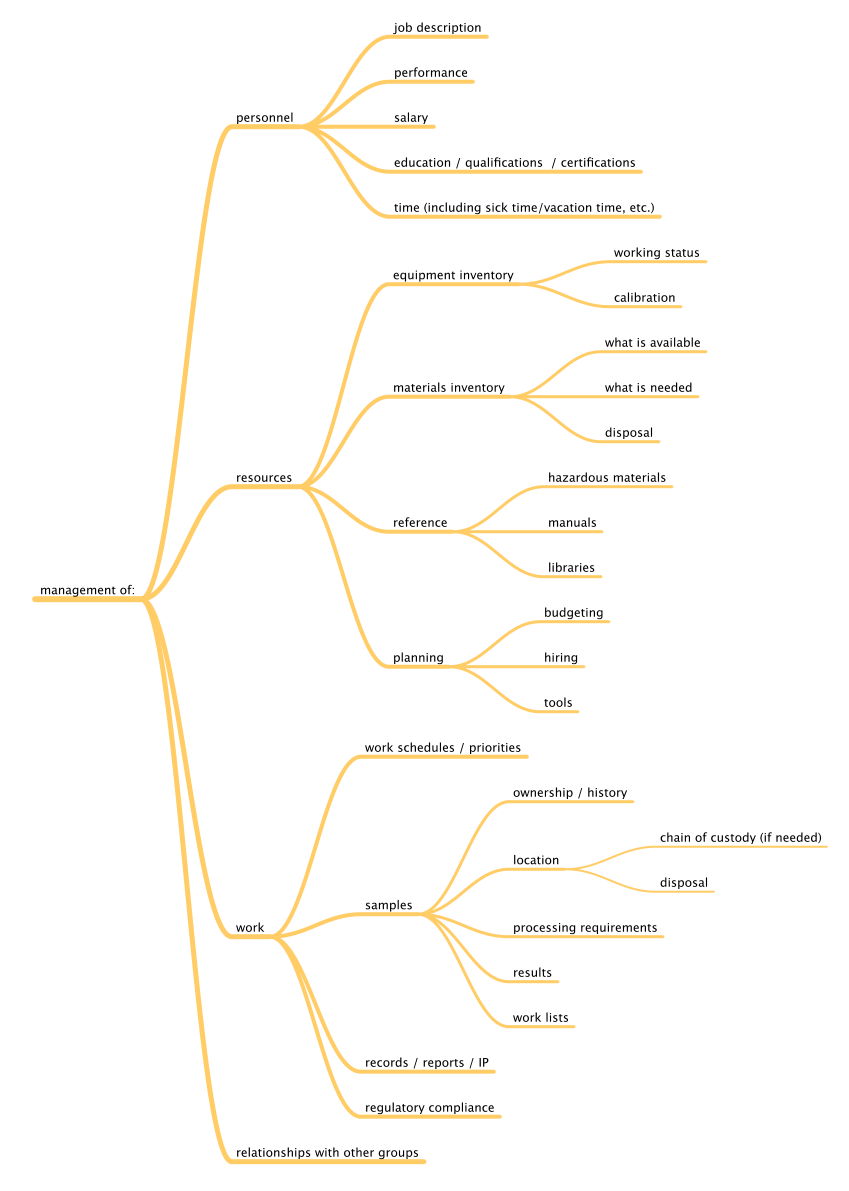
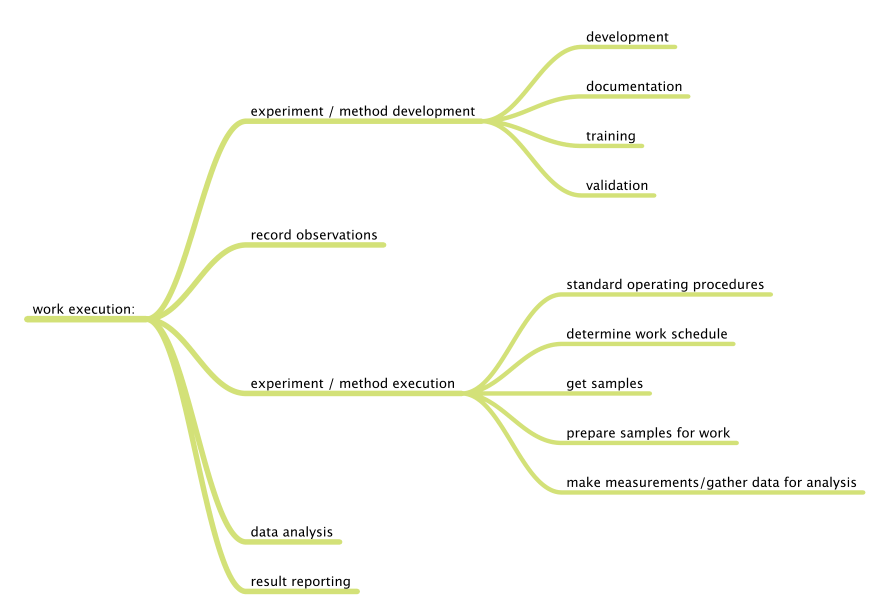
Before we can begin to understand the application of the tools and technologies that are available, we have to know what we want to accomplish, specifically what problems we want to solve. We can divide laboratory functions into two broad classes: management and work execution. Figure 1 addresses management functions, wheras Figure 2 addresses work execution functions, all common to laboratories. You can add to them based on your own experience.
|
|
Vendors have been developing products to address these work areas, and there are a lot of products available. Many of them are "point" solutions: products that are focused on one aspect of work without an effort to integrate them with others. That isn’t surprising since there isn’t an architectural basis for integration aside from specific hardware systems (e.g., Firewire, USB) or vendor-specific software systems (e.g., office product suites). Another issue in scientific work is that the vendor may only be interested in solving a particular problem, with most of the emphasis on an instrument or technique. They may provide the software needed to support their hardware, with data transfer and integration left to the user.
As you work through this document, you’ll find a map of management responsibilities and technologies. How do you connect the above map of functions to the technologies? Applying software and hardware solutions to your lab's needs requires deliberate planning. The days of purchasing point solutions to problems have passed. Today's lab managers need to think more broadly about product usage and how components of lab software systems work together. The point of this document is to help you understand what you need to think about in that regard.
Given those summaries of lab activities, how do we apply available technologies to improve lab operations? Most of the answers fall under the heading of "laboratory automation," so we’ll begin by looking at what that is.
What is laboratory automation?
This isn’t a trivial question; your answer may depend on the field you are working in, your experience, and your current interests. To some it means robotics, to others it is a LIMS (or their clinical counterpart, the laboratory information systems or LIS). The ELN and instrument data systems (IDS) are additional elements worth noting. These are examples of product classes and technologies used in lab automation, but they don’t define the field. Wikipedia provides the following as a definition[1]:
Laboratory automation is a multi-disciplinary strategy to research, develop, optimize and capitalize on technologies in the laboratory that enable new and improved processes. Laboratory automation professionals are academic, commercial and government researchers, scientists and engineers who conduct research and develop new technologies to increase productivity, elevate experimental data quality, reduce lab process cycle times, or enable experimentation that otherwise would be impossible. The most widely known application of laboratory automation technology is laboratory robotics. More generally, the field of laboratory automation comprises many different automated laboratory instruments, devices, software algorithms, and methodologies used to enable, expedite and increase the efficiency and effectiveness of scientific research in laboratories.
And McDowall offered this definition in 1993[2]:
Apparatus, instrumentation, communications or computer applications designed to mechanize or automate the whole or specific portions of the analytical process in order for a laboratory to provide timely and quality information to an organization
These definitions emphasize equipment and products, and that is where typical approaches to lab automation and the work we are doing part company. Products and technologies are important, but what is more important is figuring out how to use them effectively. The lack of consistent success in the application of lab automation technologies appears to stem from this focus on technologies and equipment—“what will this product do for my lab?”—rather than methodologies for determining what is needed and how to implement solutions.
Having a useful definition of laboratory automation is crucial since how we approach the work depends on how we see the field developing. The definition the Institute for Laboratory Automation (ILA) bases its work on is this:
Laboratory automation is the process of determining needs and requirements, planning projects and programs, evaluating products and technologies, and developing and implementing projects according to a set of methodologies that results in successful systems that increase productivity, improve the effectiveness and efficiency of laboratory operations, reduce operating costs, and provide higher-quality data.
The field includes the use of data acquisition, analysis, robotics, sample preparation, laboratory informatics, information technology, computer science, and a wide range of technologies and products from widely varying disciplines, used in the implementation of projects.
Why "process" is important
Lab automation isn’t about stuff, but how we use stuff. The “process” component of the ILA definition is central to what we do. To quote Frank Zenie[b] (one of the founders of Zymark Corporation) “you don’t automate a thing, you automate a process.” You don’t automate an instrument, you automate the process of using one. Autosamplers are a good example of successful automation: they address the process of selecting a sample vial, withdrawing fluid, positioning a needle into an injection port, injecting the sample, preparing the syringe for the next injection, and indexing the sample vials when needed.
A number of people have studied the structure of science and the relationship between disciplines. Lab automation is less about science and more about how the work of science is done. Before lab automation can be considered for a project, the underlying science has to be done. In other words, the process that automation is going to be applied to has to exist first. It also has to be the right process for consideration. This is a point that needs attention.
If you are going to spend resources on a project, you have to make sure you have a well-characterized process and that the process is both optimal and suitable for automation. This means:
- The process is well documented, people that use the process have been trained on that documented process, and their work is monitored to determine any shortcuts, workarounds, or other variations from that documented process. Differences between the published process and the one actually in use can have a significant impact on the success of a particular project design.
- The process’s “readiness for automation” has been determined. The equipment used is suitable for automation or the changes needed to make it suitable are known, and it can be done at reasonable cost. Any impact on warranties has been determined and found to be acceptable.
- If several process options exist (e.g., different test protocols for the same test question), they are evaluated for their ability to meet the needs of the science and to be successfully implemented. Other options, such as outsourcing, should be considered to make the best use of resources; is may be more cost-effective to outsource than automate.
When looking at laboratory processes, it's useful to recognize they may operate on different levels. There may be high-level processes that address the lab's reason for existence and cover the mechanics of how the lab functions, as well as low-level processes that address individual functions of the lab. This process view is important when we consider products and technologies, as the products have to fit the process, the general basis for product requirements. Discussions often revolve around LIMS and ELNs are one example, with questions being asked about whether one or both are required in the lab, or whether an ELN can replace a LIMS.
These and other similar questions reflect both vendor influence and a lack of understanding of the technologies and their application. Some differentiate the two types of technology based on “structured” (as found in LIMS) vs. “unstructured” (as found in ELNs) data. Broadly speaking, LIMS come with a well-defined, extensible, database structure, while ELNs are viewed as unstructured since you can put almost anything into an ELN and organize the contents as you see fit. But this characterization doesn’t work well either since as a user I might consider the contents, along with an index, as having a structure. This is more of an information technology approach rather than one that addresses laboratory needs. In the end, an understanding of lab processes is still required to resolve most issues.
LIMS are well-defined entities[c] and the only one of the two to carry an objective industry-standard description. LIMS are also designed to manage the processes surrounding laboratory testing in a wide variety of industries (e.g., from analytical, physical, and environmental testing, to clinical testing, which is usually associated with the LIS). The lab behavior process model is essentially the same across industries and disciplines. Basically, if you are running an analytical lab and need to manage samples and test results while answering questions about the status of testing on a larger scale than what you can memorize[d], a LIMS is a good answer.
At the time of this writing, there is no standardized definition of an ELN, though a forthcoming update of ASTM E1578 intends to recitfy that. However, given the current hype about ELNs, any product with that designation is going to get noticed. Lets avoid the term and replace it with a set of software types that addresses similar functionality:
1. Scripted execution systems – These are software systems that guide an analyst in the conduct of a procedure (process); examples include Velquest (now owned by Accelrys) products and the scripting notebook function (vendor's description) in [LabWare, Inc.|LabWare LIMS]].
2. Journal or diary system - These are software systems that provide a record of laboratory work; a word processing system might fill this need, although there are products with features specifically designed to assist lab work.
3. Application- or discipline-specific record keeping systems – These are software systems designed for biology, chemistry, mathematics and other areas that contain features that allow you to record data and text in a variety of forms that are geared toward the needs of specific areas of science.
This is not an exhaustive list of forms or functionality, but it is sufficient to make a point. The first, scripted execution, is designed around a process or, more specifically, designed to give the user a mechanism to describe the sequential steps in a process so that they can be repeated under strict controls. These do not replace a LIMS but can be used synergistically with one, or with software that duplicates LIMS capabilities (some have suggested enterprise resource planning or ERP systems as a substitute). The other two types are repositories of lab information: equations, data, details of procedures, etc. There is no general underlying process as there is with LIMS. They can provide a researcher with a means of describing experiments, collecting data, and performing analyses, which you can correctly view as processes, but they are unique to that researcher or lab and not based on any generalized industry model, as we see in testing labs.
Why is this important? These descriptions illustrate something fundamental: process dictates needs, and needs set requirements for products and technology. The “do we need a LIMS or ELN” question is meaningless without an understanding of the processes that operate in your laboratory.
The role of processes in integration
From the standpoint of equipment, laboratories are often a collection of instruments, computer systems, sample preparation stations, and other test or measurement facilities. One goal frequently stated by lab managers is that “ideally we’d like all this to be integrated.” The purpose of integration is to streamline the operations, reduce human labor, and provide a more efficient way of doing work. You are integrating the equipment and systems used to execute one or more processes. However, without a thorough evaluation of the processes in the lab, there is no basis for integration.
Highlighting the connection between processes and integration, we see why defining what laboratory automation is remains important. One definition can lead to purchasing products and limiting the scope of automation to individual tasks. Another will take you through an evaluation of how your lab works, how you want it to work, and how to produce a framework for getting you there.
The elements of laboratory technology management
Footnotes
- ↑ The Spectronic 20 was developed by Bausch & Lomb in 1954 and is currently owned and marketed in updated versions by ThermoFisher.
- ↑ Mr. Zenie often introduced robotics courses at Zymark with that statement.
- ↑ See ASTM E1578 - 06.
- ↑ This is not a recommended method.
About the author
Initially educated as a chemist, author Joe Liscouski (joe dot liscouski at gmail dot com) is an experienced laboratory automation/computing professional with over forty years of experience in the field, including the design and development of automation systems (both custom and commercial systems), LIMS, robotics and data interchange standards. He also consults on the use of computing in laboratory work. He has held symposia on validation and presented technical material and short courses on laboratory automation and computing in the U.S., Europe, and Japan. He has worked/consulted in pharmaceutical, biotech, polymer, medical, and government laboratories. His current work centers on working with companies to establish planning programs for lab systems, developing effective support groups, and helping people with the application of automation and information technologies in research and quality control environments.
References
- ↑ "Laboratory automation". Wikipedia. Archived from the original on 05 December 2014. https://en.wikipedia.org/w/index.php?title=Laboratory_automation&oldid=636846823.
- ↑ McDowall, R.D. (1993). "A Matrix for the Development of a Strategic Laboratory Information Management System". Analytical Chemistry 65 (20): 896A–901A. doi:10.1021/ac00068a725.