Difference between revisions of "User:Shawndouglas/sandbox/sublevel21"
Shawndouglas (talk | contribs) Tag: Reverted |
Shawndouglas (talk | contribs) Tag: Reverted |
||
| Line 2: | Line 2: | ||
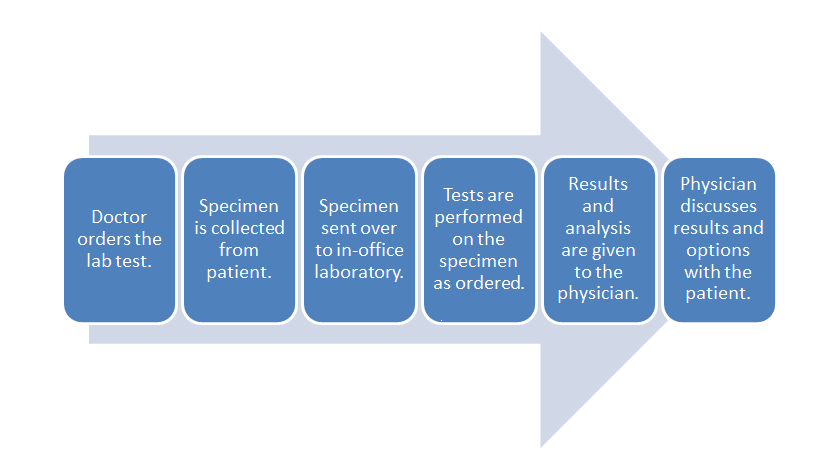
[[File:POLWorkflow.png|620px|thumb|'''Figure 5.''' This diagram depicts a generic workflow of diagnostic testing for a patient, from the doctor ordering the test, the laboratory running its tests (with its own workflow), and the doctor finally receiving the results and passing them on to the patient.]]It took three chapters, but now—with all that background in-hand—we can finally address how [[cloud computing]] relates to the various type of [[Laboratory|laboratories]] spread across multiple sectors. Let's first begin with what an average software-enabled laboratory's data [[workflow]] might look. We can then address the benefits of cloud computing within the scope of that workflow and the regulations that affect it. Afterwards, we can examine different deployment approaches and the associated costs and benefits. | [[File:POLWorkflow.png|620px|thumb|'''Figure 5.''' This diagram depicts a generic workflow of diagnostic testing for a patient, from the doctor ordering the test, the laboratory running its tests (with its own workflow), and the doctor finally receiving the results and passing them on to the patient.]]It took three chapters, but now—with all that background in-hand—we can finally address how [[cloud computing]] relates to the various type of [[Laboratory|laboratories]] spread across multiple sectors. Let's first begin with what an average software-enabled laboratory's data [[workflow]] might look. We can then address the benefits of cloud computing within the scope of that workflow and the regulations that affect it. Afterwards, we can examine different deployment approaches and the associated costs and benefits. | ||
Let's turn to a broad workflow using diagnostic testing of a patient as an example. Figure 5 depicts a generic workflow for this process, with a doctor first ordering a laboratory test for a patient. This may be done through a [[Computerized physician order entry]] (CPOE) module found in an [[electronic health record]] (EHR) system, or through some other electronic system. In turn, a test order appears in the electronic system of the laboratory. This might occur in the lab's own CPOE module, usually integrated in some way with a [[laboratory information system]] (LIS) or a [[laboratory information management system]] (LIMS). The patient then provides the necessary specimens, either at a remote location, with the specimens then getting shipped to the main lab, or directly at the lab where the testing it to be performed. Whenever the specimens are received at the laboratory, they must be checked-in or registered in the LIS or LIMS, as part of an overall responsibility of maintaining [[chain of custody]]. At this delivery point, the laboratory's own processes and workflows take over, with the LIS or LIMS aiding in the assurance that the specimens are accurately tracked from delivery to final results getting reported (and then some, if specimen retention times are necessary). | Let's turn to a broad example of laboratory workflow using diagnostic testing of a patient as an example. Figure 5 depicts a generic workflow for this process, with a doctor first ordering a laboratory test for a patient. This may be done through a [[Computerized physician order entry]] (CPOE) module found in an [[electronic health record]] (EHR) system, or through some other electronic system. In turn, a test order appears in the electronic system of the laboratory. This might occur in the lab's own CPOE module, usually integrated in some way with a [[laboratory information system]] (LIS) or a [[laboratory information management system]] (LIMS). The patient then provides the necessary specimens, either at a remote location, with the specimens then getting shipped to the main lab, or directly at the lab where the testing it to be performed. Whenever the specimens are received at the laboratory, they must be checked-in or registered in the LIS or LIMS, as part of an overall responsibility of maintaining [[chain of custody]]. At this delivery point, the laboratory's own processes and workflows take over, with the LIS or LIMS aiding in the assurance that the specimens are accurately tracked from delivery to final results getting reported (and then some, if specimen retention times are necessary). | ||
That internal laboratory workflow will look slightly different for each clinical lab, but a broad set of steps will be realized with each lab. One or more specimens get registered in the system, barcoded labels get generated, tests and any other preparative activities are scheduled for the specimens, laboratory personnel are assigned, the scheduled workloads are completed, the results get analyzed and verified, and if nothing is out of specification (OOS) or abnormal, the results get logged and placed in a deliverable report. Barring any mandated specimen retention times, the remaining specimen material is properly disposed. At this point, most of the clinical laboratory's obligations are met; the resulting report is sent to the ordering physician, who in turn shares the results with the original patient. | That internal laboratory workflow will look slightly different for each clinical lab, but a broad set of steps will be realized with each lab. One or more specimens get registered in the system, barcoded labels get generated, tests and any other preparative activities are scheduled for the specimens, laboratory personnel are assigned, the scheduled workloads are completed, the results get analyzed and verified, and if nothing is out-of-specification (OOS) or abnormal, the results get logged and placed in a deliverable report. Barring any mandated specimen retention times, the remaining specimen material is properly disposed. At this point, most of the clinical laboratory's obligations are met; the resulting report is sent to the ordering physician, who in turn shares the results with the original patient. | ||
When we look at these workflows today, a computerized component is usually associated with them. From the CPOE and EHR to the LIS and LIMS, software systems and other [[laboratory automation]] have been an increasing area of focus for improving laboratory efficiencies, reducing human error, shortening turnaround times, and improving regulatory compliance.<ref name="WeinsteinAutomation07">{{cite journal |title=Automation in the Clinical Pathology Laboratory |journal=North Carolina Medical Journal |author=Weinstein, M.; Smith, G. |volume68 |issue=2 |pages=130–31 |year=2007 |doi=10.18043/ncm.68.2.130}}</ref><ref name="PrasadTrends12">{{cite journal |title=Trends in laboratory information management system |journal=Chemometrics and Intelligent Laboratory Systems |author=Prasad, P.J.; Bodhe, G.L. |volume=118 |pages=187–92 |year=2012 |doi=10.1016/j.chemolab.2012.07.001}}</ref><ref name="CaseyDigital20">{{cite journal |title=Digital transformation risk management in forensic science laboratories |journal=Forensic Science International |author=Casey, E.; Souvignet, T.R. |volume=316 |at=110486 |year=2020 |doi=10.1016/j.forsciint.2020.110486}}</ref> In the laboratory, the LIS and LIMS are able to electronically maintain an [[audit trail]] of sample movement, analyst activities, record alteration, and much more. The modern LIS and LIMS are also able to limit access to data and functions based on assigned role, track versioning of documents, manage scheduling, issue alarms and alerts for OOS results, maintain training records, and much more. | When we look at these workflows today, a computerized component is usually associated with them. From the CPOE and EHR to the LIS and LIMS, software systems and other [[laboratory automation]] have been an increasing area of focus for improving laboratory efficiencies, reducing human error, shortening turnaround times, and improving regulatory compliance.<ref name="WeinsteinAutomation07">{{cite journal |title=Automation in the Clinical Pathology Laboratory |journal=North Carolina Medical Journal |author=Weinstein, M.; Smith, G. |volume68 |issue=2 |pages=130–31 |year=2007 |doi=10.18043/ncm.68.2.130}}</ref><ref name="PrasadTrends12">{{cite journal |title=Trends in laboratory information management system |journal=Chemometrics and Intelligent Laboratory Systems |author=Prasad, P.J.; Bodhe, G.L. |volume=118 |pages=187–92 |year=2012 |doi=10.1016/j.chemolab.2012.07.001}}</ref><ref name="CaseyDigital20">{{cite journal |title=Digital transformation risk management in forensic science laboratories |journal=Forensic Science International |author=Casey, E.; Souvignet, T.R. |volume=316 |at=110486 |year=2020 |doi=10.1016/j.forsciint.2020.110486}}</ref> In the laboratory, the LIS and LIMS are able to electronically maintain an [[audit trail]] of sample movement, analyst activities, record alteration, and much more. The modern LIS and LIMS are also able to limit access to data and functions based on assigned role, track versioning of documents, manage scheduling, issue alarms and alerts for OOS results, maintain training records, and much more. | ||
Yet with all the benefits of software use in the laboratory comes additional risks involving the security and protection of the data that software manages, transmits, and receives. Under some circumstances, these risks may become more acute by moving software systems to cloud computing environments. We broadly discussed many of those risks in the previous chapter. Data security and regulatory risk | Yet with all the benefits of software use in the laboratory comes additional risks involving the security and protection of the data that software manages, transmits, and receives. Under some circumstances, these risks may become more acute by moving software systems to cloud computing environments. We broadly discussed many of those risks in the previous chapter. Data security and regulatory risk demand that labs moving into the cloud carefully consider the appropriate and necessary use of user access controls, networking across multitenancy or shared infrastructures, and encryption and security controls offered by the cloud service provider (CSP). Technology risk demands that labs embracing cloud have staff with a constant eye on what's changing in the industry and what tools can extend the effective life span of their cloud implementations. Operational risk demands that labs integrating cloud solutions into their workflows have redundancy plans in place for how to mitigate risks from physical disasters and other threats to their data hosted in public and private clouds. Vendor risk demands that labs thoroughly vet their cloud service providers, those providers' long-term stability, and their fall-back plans should they suffer a major business loss. And finally, financial risk demands that labs turning to the cloud should have staff who are well-versed in financial management and can effectively understand the current and future costs of implementing cloud solutions in the lab. | ||
However, this talk of risk shouldn't scare away laboratory staff considering a move to the cloud; the cloud can benefit automated laboratories in many ways. However, it does require a thoughtful, organization-wide approach to managing the risks (as discussed in Chapter 3). Let's take a look at the benefits a laboratory could realize from moving to cloud-based solutions. | However, this talk of risk shouldn't scare away laboratory staff considering a move to the cloud; the cloud can benefit automated laboratories in many ways. However, it does require a thoughtful, organization-wide approach to managing the risks (as discussed in Chapter 3). Let's take a look at the benefits a laboratory could realize from moving to cloud-based solutions. | ||
Revision as of 20:19, 15 August 2023
4. Cloud computing in the laboratory
It took three chapters, but now—with all that background in-hand—we can finally address how cloud computing relates to the various type of laboratories spread across multiple sectors. Let's first begin with what an average software-enabled laboratory's data workflow might look. We can then address the benefits of cloud computing within the scope of that workflow and the regulations that affect it. Afterwards, we can examine different deployment approaches and the associated costs and benefits.
Let's turn to a broad example of laboratory workflow using diagnostic testing of a patient as an example. Figure 5 depicts a generic workflow for this process, with a doctor first ordering a laboratory test for a patient. This may be done through a Computerized physician order entry (CPOE) module found in an electronic health record (EHR) system, or through some other electronic system. In turn, a test order appears in the electronic system of the laboratory. This might occur in the lab's own CPOE module, usually integrated in some way with a laboratory information system (LIS) or a laboratory information management system (LIMS). The patient then provides the necessary specimens, either at a remote location, with the specimens then getting shipped to the main lab, or directly at the lab where the testing it to be performed. Whenever the specimens are received at the laboratory, they must be checked-in or registered in the LIS or LIMS, as part of an overall responsibility of maintaining chain of custody. At this delivery point, the laboratory's own processes and workflows take over, with the LIS or LIMS aiding in the assurance that the specimens are accurately tracked from delivery to final results getting reported (and then some, if specimen retention times are necessary).
That internal laboratory workflow will look slightly different for each clinical lab, but a broad set of steps will be realized with each lab. One or more specimens get registered in the system, barcoded labels get generated, tests and any other preparative activities are scheduled for the specimens, laboratory personnel are assigned, the scheduled workloads are completed, the results get analyzed and verified, and if nothing is out-of-specification (OOS) or abnormal, the results get logged and placed in a deliverable report. Barring any mandated specimen retention times, the remaining specimen material is properly disposed. At this point, most of the clinical laboratory's obligations are met; the resulting report is sent to the ordering physician, who in turn shares the results with the original patient.
When we look at these workflows today, a computerized component is usually associated with them. From the CPOE and EHR to the LIS and LIMS, software systems and other laboratory automation have been an increasing area of focus for improving laboratory efficiencies, reducing human error, shortening turnaround times, and improving regulatory compliance.[1][2][3] In the laboratory, the LIS and LIMS are able to electronically maintain an audit trail of sample movement, analyst activities, record alteration, and much more. The modern LIS and LIMS are also able to limit access to data and functions based on assigned role, track versioning of documents, manage scheduling, issue alarms and alerts for OOS results, maintain training records, and much more.
Yet with all the benefits of software use in the laboratory comes additional risks involving the security and protection of the data that software manages, transmits, and receives. Under some circumstances, these risks may become more acute by moving software systems to cloud computing environments. We broadly discussed many of those risks in the previous chapter. Data security and regulatory risk demand that labs moving into the cloud carefully consider the appropriate and necessary use of user access controls, networking across multitenancy or shared infrastructures, and encryption and security controls offered by the cloud service provider (CSP). Technology risk demands that labs embracing cloud have staff with a constant eye on what's changing in the industry and what tools can extend the effective life span of their cloud implementations. Operational risk demands that labs integrating cloud solutions into their workflows have redundancy plans in place for how to mitigate risks from physical disasters and other threats to their data hosted in public and private clouds. Vendor risk demands that labs thoroughly vet their cloud service providers, those providers' long-term stability, and their fall-back plans should they suffer a major business loss. And finally, financial risk demands that labs turning to the cloud should have staff who are well-versed in financial management and can effectively understand the current and future costs of implementing cloud solutions in the lab.
However, this talk of risk shouldn't scare away laboratory staff considering a move to the cloud; the cloud can benefit automated laboratories in many ways. However, it does require a thoughtful, organization-wide approach to managing the risks (as discussed in Chapter 3). Let's take a look at the benefits a laboratory could realize from moving to cloud-based solutions.
4.1 Benefits
Whether or not you fully buy into the ability of cloud computing to help you laboratory depends on a number of factors, including industry served, current data output, anticipated future data output, the regulations affecting your lab, your organization's budget, and your organization's willingness to adopt and enforce sound risk management policies and controls. A tiny material testing laboratory with relatively simple workflows and little in the way of anticipated growth in the short term may be content with using their in-house systems. A biological research group with several laboratories geographically spread across the continent creating and managing large data sets for developing new medical innovations, all while operating in a competitive environment, may see the cloud as an opportunity to grow the organization and perform more efficient work.
In fact, the biomedical sciences in general have been a good fit for cloud computing. "Omics" laboratories in particular have shown promise for cloud-based data management, with many researchers over the years demonstrating various methods of managing the "big data" networking and sharing of omics data in the cloud.[4][5][6][7] These efforts have focused on managing large data sets more efficiently while being able to securely share those data sets with researchers around the world. This data sharing—particularly while considering FAIR data principles that aim to make data findable, accessible, interoperable, and reusable[8]—is of significant benefit to research laboratories around the world; controlled access to that data via standards-based cloud computing methods certainly lends to those FAIR principles.[9]
Another area where laboratory-driven cloud computing makes sense is that of the internet of things (IoT) and networked sensors that collect data. From wearable sensors, monitors, and point-of-care diagnostic systems to wireless sensor networks, Bluetooth-enabled mobile devices, and even IoT-enabled devices and equipment directly in the laboratory, connecting those devices to cloud services and storage provides near-seamless integration of data producing instruments with laboratory workflows.[10][11] Monitoring temperature, humidity, and other ambient temperatures of freezers, fridges, and incubators while maintaining calibration and maintenance data becomes more automated.[11] Usage data on high-use instruments can be uploaded to the cloud and analyzed to enable predictive maintenance down the road.[11] And outdoor pollution monitoring systems that use low-cost, networked sensors can, upon taking a reading (a trigger event), send the result to a cloud service (at times with the help of edge computing), where a bit of uploaded code processes the data upload on-demand.[12] In all these cases, the automated collection and analysis of data using cloud components—which in turn makes that data accessible from anywhere in the world with internet access—allows laboratorians to rapidly gain advantages in how they work.
Panning outward, we see other benefits of cloud to a broad set of laboratories. Agilent Technologies, an analytical instrument developer and manufacturer, argues that the cloud can transform siloed, disparate data and information in a non-cloud, on-premises informatics solution into more actionable knowledge and wisdom, which by extension adds value to the laboratory. They also argue overall value to the lab is increased by[13]:
- "providing a higher level of connectivity and consistency for lab informatics systems and processes";
- enabling "lab managers to integrate functionality and bring context to every phase of the continuum of value, without increasing cost or risk";
- allowing IT personnel "to do more with less";
- enabling "faster, easier, more mobile access to data and tools" for lab technicians (globally);
- allowing lab leaders to reduce costs (including capital expenditure costs) and "increase team morale by enabling streamlined, self-service access to resources" ; and
- enabling labs to expand their "digital transformation initiatives," with cloud as the catalyst.
Other benefits provided to labs by cloud computing include:
- providing "the ability to easily increase or decrease their use [of infrastructure and services] as business objectives change and keep the organization nimble and competitive" (i.e., added scalability while operating more research tasks with massive data sets)[14];
- limiting responsibility of physical (in-person) access and protection of stored data to the CSP (though this comes with its own caveats concerning backing up with another provider)[15];
- limiting responsibility for technical hardware and other assets to the CSP as the organization grows and changes[15];
- inheriting the existing security protocols and compliance procedures of the provider (though again with caveats concerning vetting the CSP's security, and the inability to do so at times)[15]; and
- ensuring "that different teams are not simultaneously replicating workloads – creating greater efficiencies throughout organizations" (under the scope of the real-time update capacity of cloud globally).[16]
To sum this all up, let's look at what cloud computing is, care of Chapter 1:
an internet-based computing paradigm in which standardized and virtualized resources are used to rapidly, elastically, and cost-effectively provide a variety of globally available, "always-on" computing services to users on a continuous or as-needed basis
First, cloud technology is standardized, just like many of the techniques used in laboratories. A standardized approach to cloud computing assists with cloud services remaining compliant, which laboratories also must do. Yes, there's plenty of responsibility in ensuring all data management and use in the cloud is done so in a compliant fashion, but standardized approaches based on sound security principles help limit a laboratory's extended risk. Second, cloud technology is virtualized, meaning compute services and resources are more readily able to be recovered should system failure or disaster strike. Compare this to traditional infrastructures that inherently end up with longer periods of downtime, something which most laboratories cannot afford to have. Third, cloud services are built to be provisioned rapidly, elastically, and cost-effectively. In the case of laboratories and their workflows—especially in high-throughput labs—having scalable compute services that can be ramped up rapidly on a pay-what-you-use basis is certainly appealing to the overall business model. Finally, having those services available from anywhere with internet service, at any time, greatly expands numerous aspects of laboratory operations. Laboratorians can access data from anywhere at any time, facilitating research and discovery. Additionally, this enables remote and wireless data collection and upload from all but the most remote of locations, making environmental or even public health laboratory efforts more flexible and nimble.
- ↑ Weinstein, M.; Smith, G. (2007). "Automation in the Clinical Pathology Laboratory". North Carolina Medical Journal (2): 130–31. doi:10.18043/ncm.68.2.130.
- ↑ Prasad, P.J.; Bodhe, G.L. (2012). "Trends in laboratory information management system". Chemometrics and Intelligent Laboratory Systems 118: 187–92. doi:10.1016/j.chemolab.2012.07.001.
- ↑ Casey, E.; Souvignet, T.R. (2020). "Digital transformation risk management in forensic science laboratories". Forensic Science International 316: 110486. doi:10.1016/j.forsciint.2020.110486.
- ↑ Onsong, G.; Erdmann, J.; Spears, M.D. et al. (2014). "Implementation of Cloud based Next Generation Sequencing data analysis in a clinical laboratory". BMC Research Notes 7: 314. doi:10.1186/1756-0500-7-314. PMC PMC4036707. PMID 24885806. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4036707.
- ↑ Afgan, E.; Sloggett, C.; Goonasekera, N. et al. (2015). "Genomics Virtual Laboratory: A Practical Bioinformatics Workbench for the Cloud". PLoS One 10 (10): e0140829. doi:10.1371/journal.pone.0140829. PMC PMC4621043. PMID 26501966. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4621043.
- ↑ Ogle, C.; Reddick, D.; McKnight, C.; Biggs, T.; Pauly, R.; Ficklin, S.P.; Feltus, F.A.; Shannigrahi, S. (2021). "Named data networking for genomics data management and integrated workflows". Frontiers in Big Data 4: 582468. doi:10.3389/fdata.2021.582468. PMC PMC7968724. PMID 33748749. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7968724.
- ↑ Wilkinson, M.D.; Dumontier, M.; Aalbersberg, I.J. et al. (2016). "The FAIR Guiding Principles for scientific data management and stewardship". Scientific Data 3: 160018. doi:10.1038/sdata.2016.18. PMC PMC4792175. PMID 26978244. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4792175.
- ↑ Mons, B.; Neylon, C.; Velterop, J. et al. (2017). "Cloudy, increasingly FAIR; revisiting the FAIR Data guiding principles for the European Open Science Cloud". Information Services & Use 37 (1): 49–56. doi:10.3233/ISU-170824.
- ↑ Mayer, M.; Baeumner, A.J. (2019). "A Megatrend Challenging Analytical Chemistry: Biosensor and Chemosensor Concepts Ready for the Internet of Things". Chemical Reviews 119 (13): 7996–8027. doi:10.1021/acs.chemrev.8b00719. PMID 31070892.
- ↑ 11.0 11.1 11.2 Borfitz, D. (17 April 2020). "IoT In The Lab Includes Digital Cages And Instrument Sensors". BioIT World. https://www.bio-itworld.com/news/2020/04/17/iot-in-the-lab-includes-digital-cages-and-instrument-sensors. Retrieved 28 July 2023.
- ↑ Idrees, Z.; Zou, Z.; Zheng, L. (2018). "Edge Computing Based IoT Architecture for Low Cost Air Pollution Monitoring Systems: A Comprehensive System Analysis, Design Considerations & Development". Sensors 18 (9): 3021. doi:10.3390/s18093021. PMC PMC6163730. PMID 30201864. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6163730.
- ↑ Agilent Technologies (21 February 2019). "Cloud Adoption for Lab Informatics: Trends, Opportunities, Considerations, Next Steps" (PDF). Agilent Technologies. https://www.agilent.com/cs/library/whitepaper/public/whitepaper-cloud-adoption-openlab-5994-0718en-us-agilent.pdf. Retrieved 28 July 2023.
- ↑ Ward, S. (9 October 2019). "Cloud Computing for the Laboratory: Using data in the cloud - What it means for data security". Lab Manager. https://www.labmanager.com/cloud-computing-for-the-laboratory-736. Retrieved 28 July 2023.
- ↑ 15.0 15.1 15.2 Association of Public Health Laboratories (2017). "Breaking Through the Cloud: A Laboratory Guide to Cloud Computing" (PDF). Association of Public Health Laboratories. https://www.aphl.org/aboutAPHL/publications/Documents/INFO-2017Jun-Cloud-Computing.pdf. Retrieved 28 July 2023.
- ↑ White, R. (20 June 2023). "A Helpful Guide to Cloud Computing in a Laboratory". InterFocus Blog. InterFocus Ltd. https://www.mynewlab.com/blog/a-helpful-guide-to-cloud-computing-in-a-laboratory/. Retrieved 28 July 2023.