Difference between revisions of "LII:Planning for Disruptions in Laboratory Operations"
Shawndouglas (talk | contribs) (Saving and adding more.) |
Shawndouglas (talk | contribs) (Saving and adding more.) |
||
| Line 46: | Line 46: | ||
In today's lab, everything revolves around data and information, including its: | In today's lab, everything revolves around data and information, including its: | ||
* production (including method development and experimental planning), | *production (including method development and experimental planning), | ||
* management, | *management, | ||
* utilization, | *utilization, | ||
* analysis, | *analysis, | ||
* application (to answering questions), | *application (to answering questions), | ||
* reporting, and | *reporting, and | ||
* distribution. | *distribution. | ||
Everything we do in laboratory work addresses those points. Sometimes it is done with our hands and manual skills, while other times it takes place in the three pounds of grey matter in our heads. Stated another way, there's the work that needs to be done on-site, and the work that can be done remotely (Figure 1). | Everything we do in laboratory work addresses those points. Sometimes it is done with our hands and manual skills, while other times it takes place in the three pounds of grey matter in our heads. Stated another way, there's the work that needs to be done on-site, and the work that can be done remotely (Figure 1). | ||
| Line 60: | Line 60: | ||
{{clear}} | {{clear}} | ||
{| | {| | ||
| | | style="vertical-align:top;" | | ||
{| border="0" cellpadding="5" cellspacing="0" width="700px" | {| border="0" cellpadding="5" cellspacing="0" width="700px" | ||
|- | |- | ||
| style="background-color:white; padding-left:10px; padding-right:10px;"| <blockquote>'''Figure 1.''' Basic laboratory workflows. While this leans toward research (both pure and applied to testing), the commercial testing and QC labs can still easily be seen within this framework.</blockquote> | | style="background-color:white; padding-left:10px; padding-right:10px;" |<blockquote>'''Figure 1.''' Basic laboratory workflows. While this leans toward research (both pure and applied to testing), the commercial testing and QC labs can still easily be seen within this framework.</blockquote> | ||
|- | |- | ||
|} | |} | ||
| Line 77: | Line 77: | ||
In a situation where on-site work activities are limited due to a contagion, getting work done while minimizing people’s involvement is critical, and that means employing automation, which could take several forms. There are a few reasons for employing automation and limiting human activity in this scenario: | In a situation where on-site work activities are limited due to a contagion, getting work done while minimizing people’s involvement is critical, and that means employing automation, which could take several forms. There are a few reasons for employing automation and limiting human activity in this scenario: | ||
* Automated systems reduce error and variability in results. | *Automated systems reduce error and variability in results. | ||
* Minimizing human activity reduces the potential for disease transmission and would help maintain people’s health. | *Minimizing human activity reduces the potential for disease transmission and would help maintain people’s health. | ||
* Automated systems have a higher level of productivity and free people from mind-numbing, repetitive tasks; if there are problems finding enough people to carry out the work, we should strive to use the people we have to do things people are better suited for rather than repetitive actions. | *Automated systems have a higher level of productivity and free people from mind-numbing, repetitive tasks; if there are problems finding enough people to carry out the work, we should strive to use the people we have to do things people are better suited for rather than repetitive actions. | ||
Despite the advantages of automation in this scenario, just saying “automation can be a solution” doesn’t say much until you consider all of the ramifications of what those words mean, and what it will take to make them a reality. That’s were we’re going with this. | Despite the advantages of automation in this scenario, just saying “automation can be a solution” doesn’t say much until you consider all of the ramifications of what those words mean, and what it will take to make them a reality. That’s were we’re going with this. | ||
| Line 85: | Line 85: | ||
Before we get too deep into this, I'd like to point out that a lot of what we’ll be discussing is based on ''[[LII:Considerations in the Automation of Laboratory Procedures|Considerations in the Automation of Laboratory Procedures]]'', published in January 2021. That work notes that in order for someone to pursue the automation of a procedure or method, there are a few requirements to consider<ref name="LiscouskiConsid21">{{cite web |title=[[LII:Considerations in the Automation of Laboratory Procedures|Considerations in the Automation of Laboratory Procedures]] |author=Liscouski, J. |date=22 January 2021}}</ref>: | Before we get too deep into this, I'd like to point out that a lot of what we’ll be discussing is based on ''[[LII:Considerations in the Automation of Laboratory Procedures|Considerations in the Automation of Laboratory Procedures]]'', published in January 2021. That work notes that in order for someone to pursue the automation of a procedure or method, there are a few requirements to consider<ref name="LiscouskiConsid21">{{cite web |title=[[LII:Considerations in the Automation of Laboratory Procedures|Considerations in the Automation of Laboratory Procedures]] |author=Liscouski, J. |date=22 January 2021}}</ref>: | ||
# The method under consideration must be proven, validated, and stable (i.e., not changing, with no moving targets). | #The method under consideration must be proven, validated, and stable (i.e., not changing, with no moving targets). | ||
# The method's use in automation is predicated on there being sufficient sample throughput to make it worth doing. | #The method's use in automation is predicated on there being sufficient sample throughput to make it worth doing. | ||
# The automated procedure should be in use over a long enough period of time to justify the work and make the project financially justifiable. | #The automated procedure should be in use over a long enough period of time to justify the work and make the project financially justifiable. | ||
It's important to note that the concept of "scientific development" ends with the first consideration. In other words, method development, modification, adjustment, and add-ons end once a documented, proven process for accomplishing a lab task is fully developed. The other two considerations involve work post-scientific-development, specifically process engineering, as a sub-heading under the concept of "laboratory systems engineering" (as discussed in ''[[LII:Laboratory Technology Planning and Management: The Practice of Laboratory Systems Engineering|Laboratory Technology Planning and Management: The Practice of Laboratory Systems Engineering]]''). That's not to say that over time changes won't be needed. There may be improvements required (i.e., "evolutionary operations," as described in ''[[LII:Directions in Laboratory Systems: One Person's Perspective|Directions in Laboratory Systems: One Person's Perspective]]''), but at least get the initial system working well before you tweak it. | It's important to note that the concept of "scientific development" ends with the first consideration. In other words, method development, modification, adjustment, and add-ons end once a documented, proven process for accomplishing a lab task is fully developed. The other two considerations involve work post-scientific-development, specifically process engineering, as a sub-heading under the concept of "laboratory systems engineering" (as discussed in ''[[LII:Laboratory Technology Planning and Management: The Practice of Laboratory Systems Engineering|Laboratory Technology Planning and Management: The Practice of Laboratory Systems Engineering]]''). That's not to say that over time changes won't be needed. There may be improvements required (i.e., "evolutionary operations," as described in ''[[LII:Directions in Laboratory Systems: One Person's Perspective|Directions in Laboratory Systems: One Person's Perspective]]''), but at least get the initial system working well before you tweak it. | ||
| Line 97: | Line 97: | ||
{{clear}} | {{clear}} | ||
{| | {| | ||
| | | style="vertical-align:top;" | | ||
{| border="0" cellpadding="5" cellspacing="0" width="700px" | {| border="0" cellpadding="5" cellspacing="0" width="700px" | ||
|- | |- | ||
| style="background-color:white; padding-left:10px; padding-right:10px;"| <blockquote>'''Figure 2.''' A visualization of fully automated and manual or partially automated laboratory processes.</blockquote> | | style="background-color:white; padding-left:10px; padding-right:10px;" |<blockquote>'''Figure 2.''' A visualization of fully automated and manual or partially automated laboratory processes.</blockquote> | ||
|- | |- | ||
|} | |} | ||
| Line 114: | Line 114: | ||
{{clear}} | {{clear}} | ||
{| | {| | ||
| | | style="vertical-align:top;" | | ||
{| border="0" cellpadding="5" cellspacing="0" width="900px" | {| border="0" cellpadding="5" cellspacing="0" width="900px" | ||
|- | |- | ||
| style="background-color:white; padding-left:10px; padding-right:10px;"| <blockquote>'''Figure 3.''' Mindmap showing topics covered in this section on automation of on-site laboratory work. Numbers in parentheses match up to the subsections of this section.</blockquote> | | style="background-color:white; padding-left:10px; padding-right:10px;" |<blockquote>'''Figure 3.''' Mindmap showing topics covered in this section on automation of on-site laboratory work. Numbers in parentheses match up to the subsections of this section.</blockquote> | ||
|- | |- | ||
|} | |} | ||
| Line 123: | Line 123: | ||
===1. Is automation a realistic solution to lab productivity and reducing personnel involvement?=== | ===1. Is automation a realistic solution to lab productivity and reducing personnel involvement?=== | ||
Automation in lab work can be an effective method of addressing productivity and reducing the amount of human interaction with a process. That reduction in personnel can be advantageous in dealing with disease transmission and working with hazardous materials. But how much automation is appropriate? That question is going to be looked at throughout this discussion, but before we get into it, there are a couple of points that need to be made. | [[File:Fig4 Liscouski PlanDisruptLabOper2022.jpg|right|thumb|300px|'''Figure 4.''' A Wiley mill. Used with permission of manufacturer.]]Automation in lab work can be an effective method of addressing productivity and reducing the amount of human interaction with a process. That reduction in personnel can be advantageous in dealing with disease transmission and working with hazardous materials. But how much automation is appropriate? That question is going to be looked at throughout this discussion, but before we get into it, there are a couple of points that need to be made. | ||
Rather than discussing procedures in the abstract, we’re going to use two methods as a basis for highlighting key points. Those methods are the analysis of BHT in polyolefins, and the [[ELISA]] assay, which is widely used in the life sciences. | Rather than discussing procedures in the abstract, we’re going to use two methods as a basis for highlighting key points. Those methods are the analysis of BHT in polyolefins, and the [[ELISA]] assay, which is widely used in the life sciences. | ||
====Method 1: Analysis of antioxidants in polyolefins==== | ====Method 1: Analysis of antioxidants in polyolefins==== | ||
Antioxidants are used to prevent oxidative breakdown of polyolefins. [[wikipedia:Butylated hydroxytoluene|Butylated hydroxytoluene]] (BHT) is used at concentrations of ~1000 ppm in [[wikipedia:Polyolefin|polyolefins]] (e.g., polypropylene, polyethylene). Samples may arrive as fabricated parts or as raw materials, usually in pellets ranging in size from 1/8 to 1/4". The outline below is an abbreviated summary to highlight key points. However, the overall method is a common one for additive analysis in plastics, packaging, etc. | Antioxidants are used to prevent oxidative breakdown of polyolefins. [[wikipedia:Butylated hydroxytoluene|Butylated hydroxytoluene]] (BHT) is used at concentrations of ~1000 ppm in [[wikipedia:Polyolefin|polyolefins]] (e.g., polypropylene, polyethylene). Samples may arrive as fabricated parts or as raw materials, usually in pellets ranging in size from 1/8 to 1/4". The outline below is an abbreviated summary to highlight key points. However, the overall method is a common one for additive analysis in plastics, packaging, etc.<ref>{{Cite journal |last=Fasihnia |first=Seyedeh Homa |last2=Peighambardoust |first2=Seyed Hadi |last3=Peighambardoust |first3=Seyed Jamaleddin |last4=Oromiehie |first4=Abdulrasoul |last5=Soltanzadeh |first5=Maral |last6=Peressini |first6=Donatella |date=2020-08 |title=Migration analysis, antioxidant, and mechanical characterization of polypropylene‐based active food packaging films loaded with BHA, BHT, and TBHQ |url=https://onlinelibrary.wiley.com/doi/10.1111/1750-3841.15337 |journal=Journal of Food Science |language=en |volume=85 |issue=8 |pages=2317–2328 |doi=10.1111/1750-3841.15337 |issn=0022-1147}}</ref><ref name="NielsonOver89">{{cite web |url=https://www.waters.com/webassets/cms/library/docs/940507.pdf |format=PDF |title=Overview of Polyolefin Additive Analysis |author=Nielson, R.C. |publisher=Waters Chromatography Division |date=October 1989 |accessdate=17 March 2022}}</ref> This was a common procedure in the first lab I worked in: | ||
# Standards are prepared from high-quality BHT material in a solvent (methylene chloride, aka Dichloromethane). Note that these standards are used to calibrate the [[Chromatography|chromatograph's]] detector response to a different concentration of BHT; they are different than standard/reference samples used to monitor the process. | |||
# Samples are ground in a Wiley mill (with a 20 mesh screen) to make the material uniform in size and increase the surface area for solvent extraction (Figure 4). A Wiley mill has a hopper feed, rotating blades, and a mesh screen that controls particle size (bottom of blade chamber). The ground sample exits through the bottom into the container. As to its use, imagine making 20 cups of coffee; the beans for each cup have to be ground individually, and the grinder partially disassembled and cleaned to prevent cross-contamination. Cleaning involves brushes, air hoses, and vacuum cleaners. It makes a lot of noise. This is an example of equipment designed for human use that is difficult or impossible to include in a fully automated processing stream, and why the sample preparation discussion above is important. | |||
# Five grams of sample are weighed out and put into a flask, then 50 ml of solvent is added, and the container is sealed. | |||
# The flasks are put on a shaker for a period of time, after which they are ready for chromatographic analysis. | |||
====Method 2: ELISA, the enzyme-linked immunosorbent assay==== | |||
[[File:Fig5 Liscouski PlanDisruptLabOper2022.png|right|thumb|300px|'''Figure 5.''' A 96-well microplate, also referred to as a microtiter plate. Each well is an individual sample container.]]ELISA assays are microplate-based (ranging from 96-384 wells per plate; see Figure 5) procedures that use antibodies to detect and measure an antigen using highly specific antibody-antigen reactions. The basic outline of the assay is as follows<ref name="ThermoELISA10">{{cite web |url=http://tools.thermofisher.com/content/sfs/brochures/TR0065-ELISA-guide.pdf |format=PDF |title=ELISA technical guide and protocols |publisher=Thermo Scientific |date=2010 |accessdate=17 March 2022}}</ref><ref name="ABCAM_ELISAGuide">{{cite web |url=https://www.abcam.com/protocols/the-complete-elisa-guide |title=The complete ELISA guide |publisher=Abcam plc |date=n.d. |accessdate=17 March 2022}}</ref>: | |||
# Directly or indirectly immobilize antigens to the surface of the microplate wells. | |||
# Add an irrelevant protein or some other molecule to cover the unsaturated surface-binding portions of the microplate wells. | |||
# Incubate the microplate wells with antigen-specific antibodies that have a common affinity of binding to the antigens. | |||
# Detect the signal generated by any primary or secondary tag found on the specific antibody. | |||
Basically, a series of material additions (liquids) and washings (removal of excess material) is made in preparation for measurement. This is a procedure I’ve observed in several labs, though not personally executed. | |||
===2. Is there sufficient work to justify automation? and 3. How do you tackle automation in low-volume work environments?=== | |||
The automation of lab processes is very much like automation in any industrial process. There are two big differences, however. First, in an industrial process, management will go to considerable lengths to maximize automation efforts. Higher throughput and reduced error and variability in the results means more profits, and that—among other points like employee safety and reduced labor costs—can justify the cost of engineering automation. There is also the point that considerable effort will be made to custom design automation stations so that they will work; this is a lot different than lab situations where doing the best you can with what is available is the more common practice. Second, industrial processes tend to remain stable, varying only as technological improvements translate to higher throughput and high profitability. | |||
The impact of automation may not be as dramatic in lab systems since labs are usually classified as cost centers rather than profit centers. ([[Clinical laboratory|Clinical labs]] are one exception, where total lab automation has made a considerable impact.) Another issue is that the technologies used in lab instruments and systems are developed as independent entities, designed to do a particular task, with little concern as to how they fit into a lab's specific procedures. It’s up to the lab to make things work by putting components together in sequence, at least for one generation. However, engineering systems need to operate over the long term, preferably with built-in upgrade facilities, something that usually hasn't been considered in lab automation work. One departure from this are the automation systems built upon microplate technologies (e.g., ELISA). Components from varied vendors have to meet the requirements of microplate geometries and thus work together via robotics automation or manual effort (i.e., people acting as the robots). | |||
As an example, industrial production lines rarely if ever come into being without precursors. Manufacturing something starts with people carrying out tasks that may be assisted by machinery, and gradually a production line develops, with multiple people doing specialized work that we would recognize as a production line. As demand for products increases, higher productivity is needed. Automation is added until we have a fully automated production facility. The development of this automation is based on justified need; there is sufficient work to warrant its implementation. | |||
Laboratories are no different in this regard. Procedures are executed manually until the process is worked out and proven, and eventually the lab can look to gradually improving throughput and efficiency as the volume of work begins to increase. The leads to several approaches to dealing with increasing volume. | |||
Looking at Method 1, the following automation additions may take place over time: | |||
* Manual injection of samples/standards/references into the chromatograph gives way to an autosampler. | |||
* Manual analysis of chromatograms gives way to a [[chromatography data system]] (CDS). (More on this in part 6.) | |||
# The extraction and mixing stages could be automated using a device similar to the Agilent 7693A automatic liquid sampler.<ref name="AgilentHas21">{{cite web |url=https://www.agilent.com/cs/library/brochures/5991-1287EN-Autosampler_portfolio_brochure.pdf |format=PDF |title=Agilent Has The Right Sample Introduction Product For Your Lab |publisher=Agilent |date=15 March 2021 |accessdate=17 March 2022}}</ref> This would use a much smaller amount of sample and solvent, though sensitivity could become an issue, as could small particles of ground sample blocking or entering the syringe. | |||
Revision as of 23:05, 17 March 2022
Title: Planning for Disruptions in Laboratory Operations
Author for citation: Joe Liscouski, with editorial modifications by Shawn Douglas
License for content: Creative Commons Attribution-ShareAlike 4.0 International
Publication date: March 2022
|
|
This article should be considered a work in progress and incomplete. Consider this article incomplete until this notice is removed. |
Introduction
A high-level of productivity is something laboratory management wants and those working for them strive to achieve. However, what happens when reality trips us up? We found out when COVID-19 appeared.
This work examines how laboratory operations can be organized to meet that disruption, as well as others we may have to face. Many of these changes, including the introduction of new technologies and changing attitudes about work, were in the making already but at a much slower pace.
A brief look at "working"
Over the years, productivity has had many measures, from 40 to 60 hour work weeks and piece-work to pounds of material processed to samples run, all of which comes from a manufacturing mind set. People went to work in an office, lab, or production site, did their work, put in their time, and went home. That was in the timeframe leading up to the 1950s and '60s. Today, in 2022, things have changed.
People went to a work site because that’s where the work, and the tools they needed to do it, were, along with the people they needed to interact with. Secure electronic communications changed all that. As long as carrying out your work depended on specialized, fixed-in-place equipment, you went to the work site. Once it became portable, doing the work depended on where you were and the ability to connect with those you worked with.
The activity of working was a normal, routine thing. Changes in the way operations were carried out were a function of the adoption of new technologies and practices, i.e., normal organizational evolution. However, just as in the development of living systems, the organizational evolutionary process will eventually face a new challenge that throws work operations into disorder. It's a given that disruptions in lab operations are going to occur, and you need to be prepared to meet them.
To that point, the emergence of COVID-19 in our society has accelerated shifts in organizational behavior that might otherwise have taken a decade to develop. That order of magnitude of increase in the rate of change exposed both opportunities people were able to take advantage of and understandable gaps that a slower pace may have planned for. We need to look at what we have learned in responding to the constraints imposed by the pandemic, how we can prepare for the continuation of its impact, and how we can take advantage of technologies to adapt to new ways of conducting scientific work.
This work is not just a historical curiosity but an examination of what we will have to do to meet the challenges of emerging transmissible diseases and geographical population fragmentation caused by, for example, climate change (e.g., disruptions due to storms, power outages, difficulties traveling, etc.) and people’s mobility. All that doesn’t begin to take into account difficulties in retaining personnel and hiring new people.
Much of the “mobility” issue (i.e., “we can work from anywhere”) comes from the idea of “knowledge workers” in office environments and doesn’t apply to manufacturing and lab bench work. Yes, lab work is also knowledge-based, but it’s execution may not be portable, depending on the equipment used and regulatory restrictions (corporate or otherwise) that might be in place.
Why does this matter?
A number of articles have detailed how the COVID-driven shift to remote and hybrid working environments has changed people's attitudes about work, focusing on office activities: what they do and how they do it. Some of it is a change in balance between work and personal lives, some a recognition that “the way things have always been done” isn’t the way things have to be. Where possible some people would like choices in working conditions. That isn’t universal.
Writing for Fortune, journalist Geoff Colvin notes that people who are driven to be fast-track successful prefer to return to a traditional office work environment where closer contact between management and employees can occur; their efforts are more visible.[1] Granted those surveyed for Colvin's article are in financial companies, and are perhaps more driven by shorter term advancement. In addition, a second article written by Erica Pandey for Axios shows that the laboratory real estate market is “hot” and growing. More importantly it is growing where lab-wise intelligence is concentrating, places where similar working environments exist, as well as educational opportunities.[2] That means a growing job market with opportunities for growth and change, but potentially in a confined set of geographies that are vulnerable to problems noted earlier. How do we relieve that potential stress? What happens when severe weather, power disruptions, and contagious disease outbreaks[3] push against concentrations of people working in tight quarters? Is the lab environment flexible enough to adapt to changes in how work gets done?
We need to look at what “lab work” is and where it differs from other forms of work, and where it is similar.
The nature of laboratory work
There are two aspects to working in a laboratory: meeting goals that are important to your organization, and meeting goals that are important to you, both professionally and personally. After all, productivity isn’t the only measure of satisfaction with lab work; people’s satisfaction with their work is also very important.
When you think of lab operations, what functions come to mind? It's relatively easy to think of experiments, analysis, reporting, and planning the next set of experiments. Some may even consider the supply chain and waste disposal as part of laboratory operations. But what of data and information management practices in the lab?
In today's lab, everything revolves around data and information, including its:
- production (including method development and experimental planning),
- management,
- utilization,
- analysis,
- application (to answering questions),
- reporting, and
- distribution.
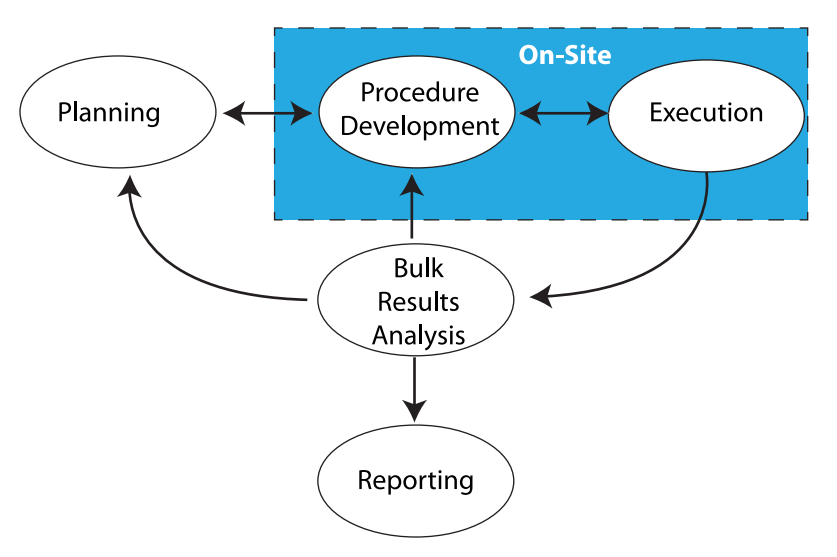
Everything we do in laboratory work addresses those points. Sometimes it is done with our hands and manual skills, while other times it takes place in the three pounds of grey matter in our heads. Stated another way, there's the work that needs to be done on-site, and the work that can be done remotely (Figure 1).
|
The work that needs to be done on-site is found in the blue box in Figure 1, consisting of benchtop work, instrumentation, materials preparation, support equipment, etc. This is the stuff people usually think about when “lab work” is mentioned. Aside from equipment that is designed to be portable and made for field use, most laboratory equipment isn’t able to be relocated without a lot of time-consuming tear-down and set-up work, as well as proving that the equipment/instrumentation is operating properly according to specifications for use afterwards. In addition, there are concerns with materials (e.g., solvents, etc.) that need to be used, their proper handing, storage, and disposal. That isn’t to say that transportable instrumentation is possible; mobile labs exist and work well, but because they are designed to. Those are special cases and not representative of the routine lab setting.
The space outside the blue box in Figure 1 consists of thinking, meetings, and working with documents, models, analytics, etc. That work should be portable and may only be restricted by policies concerning external access to laboratory/corporate systems, as well as those governing the removal of corporate property (e.g., intellectual property and computers) from the normal work site. Permitting/restricting external access isn’t a trivial point; if you can get access to information from outside the lab, so can someone else unless security measures are carefully planned.
The next section will focus on addressing laboratory work in the age of COVID-19 and other transmissible diseases. To be sure, the SARS-CoV-2 virus and its variants are just the immediate concern; other infectious elements may manifest themselves as a normal part of people interacting with the world, as well as by releases of disease resulting from climate change. There are other disruptive events that need to be addressed, and they will be covered later.
On-site work and automation: A preview
In a situation where on-site work activities are limited due to a contagion, getting work done while minimizing people’s involvement is critical, and that means employing automation, which could take several forms. There are a few reasons for employing automation and limiting human activity in this scenario:
- Automated systems reduce error and variability in results.
- Minimizing human activity reduces the potential for disease transmission and would help maintain people’s health.
- Automated systems have a higher level of productivity and free people from mind-numbing, repetitive tasks; if there are problems finding enough people to carry out the work, we should strive to use the people we have to do things people are better suited for rather than repetitive actions.
Despite the advantages of automation in this scenario, just saying “automation can be a solution” doesn’t say much until you consider all of the ramifications of what those words mean, and what it will take to make them a reality. That’s were we’re going with this.
Before we get too deep into this, I'd like to point out that a lot of what we’ll be discussing is based on Considerations in the Automation of Laboratory Procedures, published in January 2021. That work notes that in order for someone to pursue the automation of a procedure or method, there are a few requirements to consider[4]:
- The method under consideration must be proven, validated, and stable (i.e., not changing, with no moving targets).
- The method's use in automation is predicated on there being sufficient sample throughput to make it worth doing.
- The automated procedure should be in use over a long enough period of time to justify the work and make the project financially justifiable.
It's important to note that the concept of "scientific development" ends with the first consideration. In other words, method development, modification, adjustment, and add-ons end once a documented, proven process for accomplishing a lab task is fully developed. The other two considerations involve work post-scientific-development, specifically process engineering, as a sub-heading under the concept of "laboratory systems engineering" (as discussed in Laboratory Technology Planning and Management: The Practice of Laboratory Systems Engineering). That's not to say that over time changes won't be needed. There may be improvements required (i.e., "evolutionary operations," as described in Directions in Laboratory Systems: One Person's Perspective), but at least get the initial system working well before you tweak it.
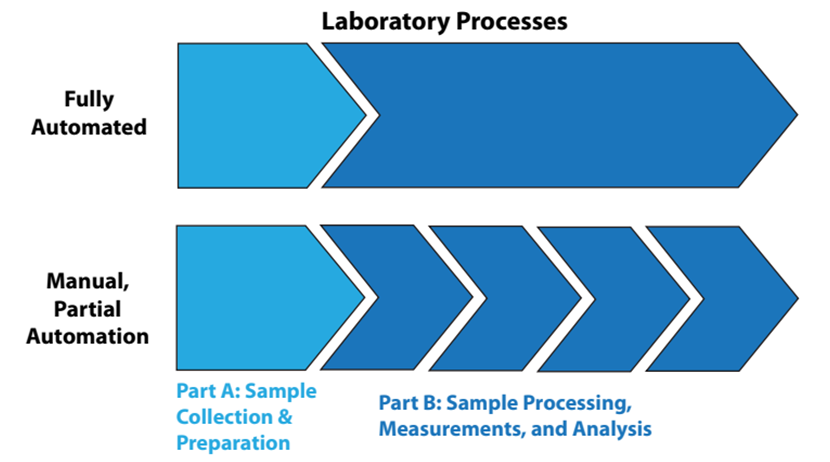
That said, let's look at the automation of lab processes from two viewpoints: “fully” automated laboratory processes and manually or partially automated lab processes, as shown in Figure 2.
|
In fully automated processes, the items in Part B of Figure 2 are a continuous sequence of events, without human intervention. In the lower portion of the illustration, Part B is a sequence of disconnected steps, usually connected by manual effort. Figure 2 might seem simplistic and obvious, but it is there to emphasize a point: sample collection and preparation are often separated from automated processing. Unless the samples have characteristics that make them easy to handle (e.g., fluids, free flowing powders), preparation of the samples for analysis is usually a time-consuming manual effort that can frustrate the benefits of automation. The same goes for sample storage and retrieval. The solution to these issues will vary from industry to industry, but they are worth addressing since good solutions would considerably improve the operational efficiency of labs and reduce manual labor requirements.
Preventing disruptions through the automation of on-site laboratory work
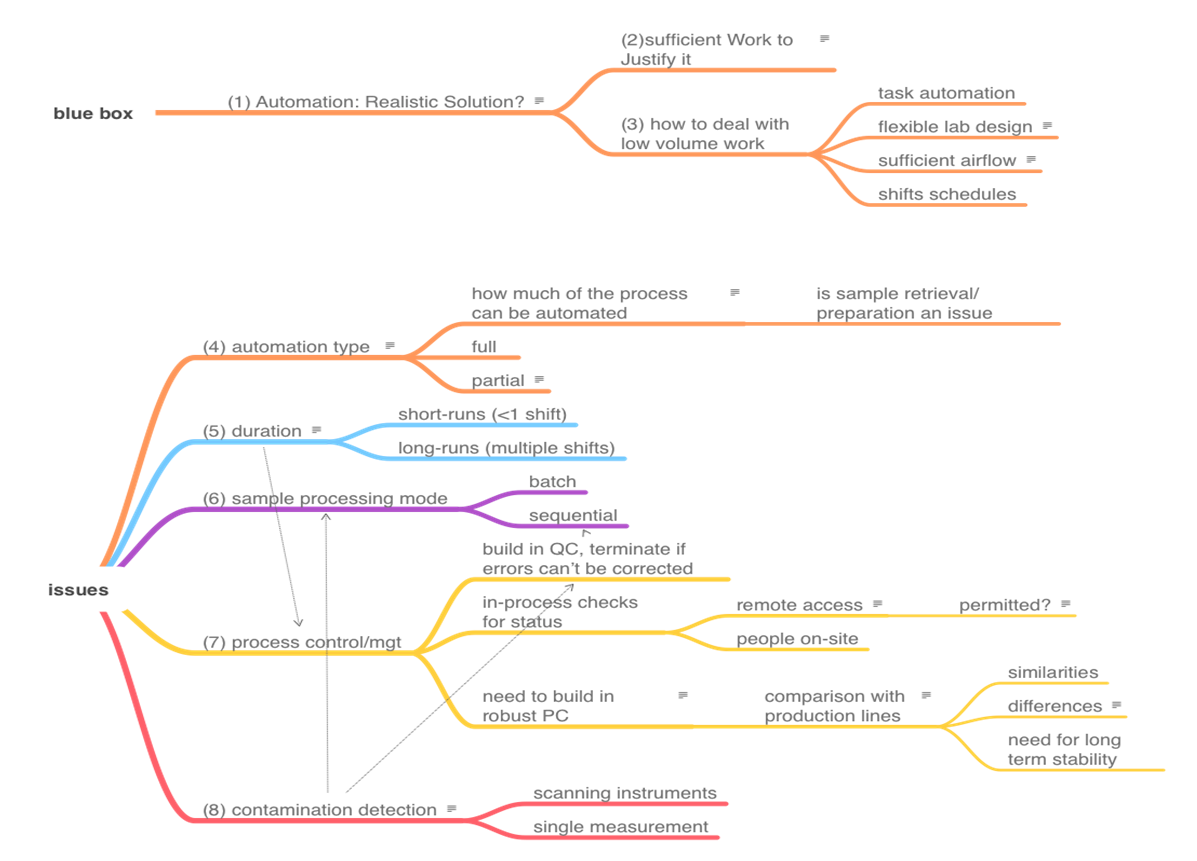
The topics that we need to cover in order to address the automation of on-site work are shown in the mindmap in Figure 3. There is no reasonable way to present the topics as a smooth narrative since they are heavily interrelated. View this mindmap as the organizing feature or "table of contents" of this section, and then we'll cover each item. The numbers in parentheses in Figure 3 represent subsection numbers, intended to make finding things easier.
|
1. Is automation a realistic solution to lab productivity and reducing personnel involvement?
Automation in lab work can be an effective method of addressing productivity and reducing the amount of human interaction with a process. That reduction in personnel can be advantageous in dealing with disease transmission and working with hazardous materials. But how much automation is appropriate? That question is going to be looked at throughout this discussion, but before we get into it, there are a couple of points that need to be made.
Rather than discussing procedures in the abstract, we’re going to use two methods as a basis for highlighting key points. Those methods are the analysis of BHT in polyolefins, and the ELISA assay, which is widely used in the life sciences.
Method 1: Analysis of antioxidants in polyolefins
Antioxidants are used to prevent oxidative breakdown of polyolefins. Butylated hydroxytoluene (BHT) is used at concentrations of ~1000 ppm in polyolefins (e.g., polypropylene, polyethylene). Samples may arrive as fabricated parts or as raw materials, usually in pellets ranging in size from 1/8 to 1/4". The outline below is an abbreviated summary to highlight key points. However, the overall method is a common one for additive analysis in plastics, packaging, etc.[5][6] This was a common procedure in the first lab I worked in:
- Standards are prepared from high-quality BHT material in a solvent (methylene chloride, aka Dichloromethane). Note that these standards are used to calibrate the chromatograph's detector response to a different concentration of BHT; they are different than standard/reference samples used to monitor the process.
- Samples are ground in a Wiley mill (with a 20 mesh screen) to make the material uniform in size and increase the surface area for solvent extraction (Figure 4). A Wiley mill has a hopper feed, rotating blades, and a mesh screen that controls particle size (bottom of blade chamber). The ground sample exits through the bottom into the container. As to its use, imagine making 20 cups of coffee; the beans for each cup have to be ground individually, and the grinder partially disassembled and cleaned to prevent cross-contamination. Cleaning involves brushes, air hoses, and vacuum cleaners. It makes a lot of noise. This is an example of equipment designed for human use that is difficult or impossible to include in a fully automated processing stream, and why the sample preparation discussion above is important.
- Five grams of sample are weighed out and put into a flask, then 50 ml of solvent is added, and the container is sealed.
- The flasks are put on a shaker for a period of time, after which they are ready for chromatographic analysis.
Method 2: ELISA, the enzyme-linked immunosorbent assay
ELISA assays are microplate-based (ranging from 96-384 wells per plate; see Figure 5) procedures that use antibodies to detect and measure an antigen using highly specific antibody-antigen reactions. The basic outline of the assay is as follows[7][8]:
- Directly or indirectly immobilize antigens to the surface of the microplate wells.
- Add an irrelevant protein or some other molecule to cover the unsaturated surface-binding portions of the microplate wells.
- Incubate the microplate wells with antigen-specific antibodies that have a common affinity of binding to the antigens.
- Detect the signal generated by any primary or secondary tag found on the specific antibody.
Basically, a series of material additions (liquids) and washings (removal of excess material) is made in preparation for measurement. This is a procedure I’ve observed in several labs, though not personally executed.
2. Is there sufficient work to justify automation? and 3. How do you tackle automation in low-volume work environments?
The automation of lab processes is very much like automation in any industrial process. There are two big differences, however. First, in an industrial process, management will go to considerable lengths to maximize automation efforts. Higher throughput and reduced error and variability in the results means more profits, and that—among other points like employee safety and reduced labor costs—can justify the cost of engineering automation. There is also the point that considerable effort will be made to custom design automation stations so that they will work; this is a lot different than lab situations where doing the best you can with what is available is the more common practice. Second, industrial processes tend to remain stable, varying only as technological improvements translate to higher throughput and high profitability.
The impact of automation may not be as dramatic in lab systems since labs are usually classified as cost centers rather than profit centers. (Clinical labs are one exception, where total lab automation has made a considerable impact.) Another issue is that the technologies used in lab instruments and systems are developed as independent entities, designed to do a particular task, with little concern as to how they fit into a lab's specific procedures. It’s up to the lab to make things work by putting components together in sequence, at least for one generation. However, engineering systems need to operate over the long term, preferably with built-in upgrade facilities, something that usually hasn't been considered in lab automation work. One departure from this are the automation systems built upon microplate technologies (e.g., ELISA). Components from varied vendors have to meet the requirements of microplate geometries and thus work together via robotics automation or manual effort (i.e., people acting as the robots).
As an example, industrial production lines rarely if ever come into being without precursors. Manufacturing something starts with people carrying out tasks that may be assisted by machinery, and gradually a production line develops, with multiple people doing specialized work that we would recognize as a production line. As demand for products increases, higher productivity is needed. Automation is added until we have a fully automated production facility. The development of this automation is based on justified need; there is sufficient work to warrant its implementation.
Laboratories are no different in this regard. Procedures are executed manually until the process is worked out and proven, and eventually the lab can look to gradually improving throughput and efficiency as the volume of work begins to increase. The leads to several approaches to dealing with increasing volume.
Looking at Method 1, the following automation additions may take place over time:
- Manual injection of samples/standards/references into the chromatograph gives way to an autosampler.
- Manual analysis of chromatograms gives way to a chromatography data system (CDS). (More on this in part 6.)
- The extraction and mixing stages could be automated using a device similar to the Agilent 7693A automatic liquid sampler.[9] This would use a much smaller amount of sample and solvent, though sensitivity could become an issue, as could small particles of ground sample blocking or entering the syringe.
References
- ↑ Colvin, G. (3 December 2021). "Why employers offering lavish work-from-home perks could be making a strategic blunder". Fortune. Archived from the original on 07 December 2021. https://web.archive.org/web/20211207025431/https://fortune.com/2021/12/03/remote-work-from-home-office-attracting-talent-productivity/. Retrieved 16 March 2022.
- ↑ Pandey, E. (7 December 2021). "Exploding demand for laboratory space". Axios. https://www.axios.com/demand-for-lab-office-space-rising-cbre-f45b20dd-8adf-48d9-a728-b43f7bb8b676.html. Retrieved 16 March 2022.
- ↑ Fox, M. (9 December 2021). "The world is unprepared for the next pandemic, study finds". CNN Health. https://www.cnn.com/2021/12/08/health/world-unprepared-pandemic-report/index.html. Retrieved 16 March 2022.
- ↑ Liscouski, J. (22 January 2021). "Considerations in the Automation of Laboratory Procedures".
- ↑ Fasihnia, Seyedeh Homa; Peighambardoust, Seyed Hadi; Peighambardoust, Seyed Jamaleddin; Oromiehie, Abdulrasoul; Soltanzadeh, Maral; Peressini, Donatella (1 August 2020). "Migration analysis, antioxidant, and mechanical characterization of polypropylene‐based active food packaging films loaded with BHA, BHT, and TBHQ" (in en). Journal of Food Science 85 (8): 2317–2328. doi:10.1111/1750-3841.15337. ISSN 0022-1147. https://onlinelibrary.wiley.com/doi/10.1111/1750-3841.15337.
- ↑ Nielson, R.C. (October 1989). "Overview of Polyolefin Additive Analysis" (PDF). Waters Chromatography Division. https://www.waters.com/webassets/cms/library/docs/940507.pdf. Retrieved 17 March 2022.
- ↑ "ELISA technical guide and protocols" (PDF). Thermo Scientific. 2010. http://tools.thermofisher.com/content/sfs/brochures/TR0065-ELISA-guide.pdf. Retrieved 17 March 2022.
- ↑ "The complete ELISA guide". Abcam plc. 31 January 2026. https://www.abcam.com/protocols/the-complete-elisa-guide. Retrieved 17 March 2022.
- ↑ "Agilent Has The Right Sample Introduction Product For Your Lab" (PDF). Agilent. 15 March 2021. https://www.agilent.com/cs/library/brochures/5991-1287EN-Autosampler_portfolio_brochure.pdf. Retrieved 17 March 2022.