Difference between revisions of "Journal:PCM-SABRE: A platform for benchmarking and comparing outcome prediction methods in precision cancer medicine"
Shawndouglas (talk | contribs) (Created stub. Saving and adding more.) |
Shawndouglas (talk | contribs) (Saving and adding more.) |
||
| Line 38: | Line 38: | ||
Efforts to stratify patients by risk of recurrence in other tumor types, and the ability to stratify patients by overall chances of survival are not as advanced. Moreover, the relative success in risk stratification for breast cancer patients has been challenged<ref name="SenkusPrimary15">{{cite journal |title=Primary breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up |journal=Annals of Oncology |author=Senkus, E.; Kyriakides, S.; Ohno, S. et al. |volume=26 |issue=Suppl 5 |pages=v8-30 |year=2015 |doi=10.1093/annonc/mdv298 |pmid=26314782}}</ref>, proposing that it in fact stratifies patients into tumor subtypes, which can be achieved with much simpler tests. | Efforts to stratify patients by risk of recurrence in other tumor types, and the ability to stratify patients by overall chances of survival are not as advanced. Moreover, the relative success in risk stratification for breast cancer patients has been challenged<ref name="SenkusPrimary15">{{cite journal |title=Primary breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up |journal=Annals of Oncology |author=Senkus, E.; Kyriakides, S.; Ohno, S. et al. |volume=26 |issue=Suppl 5 |pages=v8-30 |year=2015 |doi=10.1093/annonc/mdv298 |pmid=26314782}}</ref>, proposing that it in fact stratifies patients into tumor subtypes, which can be achieved with much simpler tests. | ||
As a result, a large number of papers have been published and are still being published where gene expression data is analyzed in order to construct models that predict cancer survival or cancer recurrence. Much of these efforts are concentrated on breast cancer, the second most commonly diagnosed cancer among American women (besides skin cancer).<ref name="BCOrgUSBreast16">{{cite web |url=http://www.breastcancer.org/symptoms/understand_bc/statistics |title=U.S. Breast Cancer Statistics |publisher=Breastcancer.org |date=2016 |accessdate=20 December 2016}}</ref> About 1 in 8 U.S. women (about 12 percent) will develop invasive breast cancer over the course of her lifetime, and similar rates are reported worldwide.<ref name="WCRFBreast16">{{cite web |url=http://www.wcrf.org/int/cancer-facts-figures/data-specific-cancers/breast-cancer-statistics |title=Breast cancer statistics |publisher=World Cancer Research Fund International |date=2016 |accessdate=20 December 2016}}</ref> Breast cancer is an attractive domain for risk stratification as it is estimated that resection is a sufficient treatment for 70 to 80 percent of the patients, while the remaining patients will develop advanced metastatic lesions, which are largely impossible to cure.<ref name="MBCNStatistics16">{{cite web |url=http://www.mbcn.org/statistics-for-metastatic-breast-cancer/ |title=Statistics for Metastatic Breast Cancer |publisher=Metastatic Breast Cancer Network |date=2016 |accessdate=20 December 2016}}</ref> Aggressive chemotherapy will reduce the chance of advanced metastasis for those patients in that situation, though it would be harmful and unnecessary therapy for those who aren't. Thus, great efforts have been invested in stratifying patients’ risk of recurrence. | As a result, a large number of papers have been published and are still being published where gene expression data is analyzed in order to construct models that predict cancer survival or cancer recurrence. Much of these efforts are concentrated on breast cancer, the second most commonly diagnosed cancer among American women (besides skin cancer).<ref name="BCOrgUSBreast16">{{cite web |url=http://www.breastcancer.org/symptoms/understand_bc/statistics |title=U.S. Breast Cancer Statistics |publisher=Breastcancer.org |date=2016 |accessdate=20 December 2016}}</ref> About 1 in 8 U.S. women (about 12 percent) will develop invasive breast cancer over the course of her lifetime, and similar rates are reported worldwide.<ref name="WCRFBreast16">{{cite web |url=http://www.wcrf.org/int/cancer-facts-figures/data-specific-cancers/breast-cancer-statistics |title=Breast cancer statistics |publisher=World Cancer Research Fund International |date=2016 |accessdate=20 December 2016}}</ref> Breast cancer is an attractive domain for risk stratification as it is estimated that resection is a sufficient treatment for 70 to 80 percent of the patients, while the remaining patients will develop advanced metastatic lesions, which are largely impossible to cure.<ref name="MBCNStatistics16">{{cite web |url=http://www.mbcn.org/statistics-for-metastatic-breast-cancer/ |title=Statistics for Metastatic Breast Cancer |publisher=Metastatic Breast Cancer Network |date=2016 |accessdate=20 December 2016}}</ref> Aggressive chemotherapy will reduce the chance of advanced metastasis for those patients in that situation, though it would be harmful and unnecessary therapy for those who aren't. Thus, great efforts have been invested in stratifying patients’ risk of recurrence.<ref name="CroninAnalytical07">{{cite journal |title=Analytical validation of the Oncotype DX genomic diagnostic test for recurrence prognosis and therapeutic response prediction in node-negative, estrogen receptor-positive breast cancer |journal=Clinical Chemistry |author=Cronin, M.; Sangli, C.; Liu, M.L. et al. |volume=53 |issue=6 |pages=1084-91 |year=2007 |doi=10.1373/clinchem.2006.076497 |pmid=17463177}}</ref> | ||
Due to the importance of risk stratification in breast cancer, combined with its relatively high abundance, breast cancer is the type of tumor for which expression profiles of newly diagnosed patients are most abundant. Several works have been published that apply machine-learning techniques to this data for predicting cancer survivability.<ref name="GyörffyAnOnline10">{{cite journal |title=An online survival analysis tool to rapidly assess the effect of 22,277 genes on breast cancer prognosis using microarray data of 1,809 patients |journal=Breast Cancer Research and Treatment |author=Györffy, B.; Lanczky, A.; Eklund, A.C. et al. |volume=123 |issue=3 |pages=725-31 |year=2010 |doi=10.1007/s10549-009-0674-9 |pmid=20020197}}</ref><ref name="NaoiDevelopment11">{{cite journal |title=Development of 95-gene classifier as a powerful predictor of recurrences in node-negative and ER-positive breast cancer patients |journal=Breast Cancer Research and Treatment |author=Naoi, Y.; Kishi, K.; Tanei, T. et al. |volume=128 |issue=3 |pages=633-41 |year=2011 |doi=10.1007/s10549-010-1145-z |pmid=20803240}}</ref> Unfortunately, we found it quite challenging to directly compare these works for the following reasons: | Due to the importance of risk stratification in breast cancer, combined with its relatively high abundance, breast cancer is the type of tumor for which expression profiles of newly diagnosed patients are most abundant. Several works have been published that apply machine-learning techniques to this data for predicting cancer survivability.<ref name="GyörffyAnOnline10">{{cite journal |title=An online survival analysis tool to rapidly assess the effect of 22,277 genes on breast cancer prognosis using microarray data of 1,809 patients |journal=Breast Cancer Research and Treatment |author=Györffy, B.; Lanczky, A.; Eklund, A.C. et al. |volume=123 |issue=3 |pages=725-31 |year=2010 |doi=10.1007/s10549-009-0674-9 |pmid=20020197}}</ref><ref name="NaoiDevelopment11">{{cite journal |title=Development of 95-gene classifier as a powerful predictor of recurrences in node-negative and ER-positive breast cancer patients |journal=Breast Cancer Research and Treatment |author=Naoi, Y.; Kishi, K.; Tanei, T. et al. |volume=128 |issue=3 |pages=633-41 |year=2011 |doi=10.1007/s10549-010-1145-z |pmid=20803240}}</ref> Unfortunately, we found it quite challenging to directly compare these works for the following reasons: | ||
| Line 49: | Line 49: | ||
==Implementation== | ==Implementation== | ||
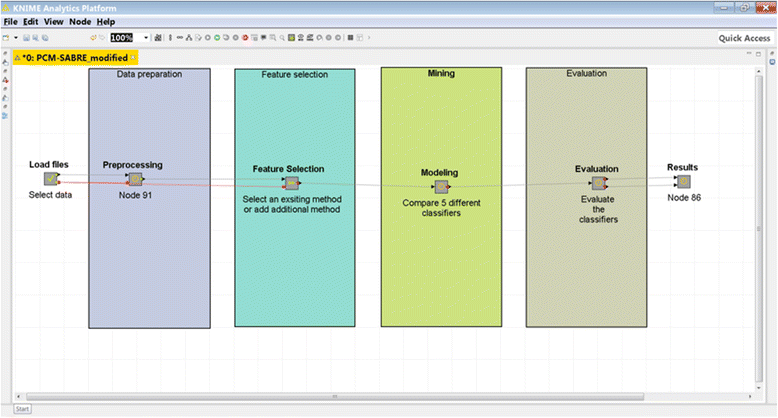
We developed a platform that allows replicating, comparing and improving knowledge discovery pipelines for cancer survival predictions, and demonstrate its applicability for Breast Cancer (Fig. 1). PCM-SABRE (Precision Cancer Medicine - Survival Analysis Benchmarking, Reporting and Evaluation), was developed using [[KNIME]] (Konstanz Information Miner).<ref name="BertholdKNIME08">{{cite book |url=http://www.inf.uni-konstanz.de/bioml2/publications/Papers2007/BCDG+07_knime_gfkl.pdf |format=PDF |chapter=KNIME: The Konstanz Information Miner |title=Data Analysis, Machine Learning and Applications |author=Berthold, M.R.; Cebron, N.; Dill, F. et al. |editor=Preisach, C.; Burkhardt, H.; Schmidt-Thieme, L.; Decker, R. |publisher=Springer-Verlag Berlin Heidelberg |pages=319–326 |year=2008 |isbn=9783540782469 |doi=10.1007/978-3-540-78246-9}}</ref> KNIME is a modern, flexible and intuitive open-source data analytics platform that allows performing sophisticated statistics and data mining analysis to develop, among other things, predictive models. We chose KNIME since it is a popular, user-friendly software that does not require programing knowledge. Its node-based workflow structure allows easily assessing the impact of changing one knowledge discovery step (for example, data mining algorithm) on the predictive performance without changing any other steps of the workflow. Another major benefit of KNIME is the ability to create new nodes; this feature is particularly useful when a researcher is interested in integrating a new method he developed into an existing KNIME workflow. | |||
[[File:Fig1 Eyal-Altman BMCBioinformatics2017 18.gif|778px]] | |||
{{clear}} | |||
{| | |||
| STYLE="vertical-align:top;"| | |||
{| border="0" cellpadding="5" cellspacing="0" width="778px" | |||
|- | |||
| style="background-color:white; padding-left:10px; padding-right:10px;"| <blockquote>'''Figure 1.''' Screenshot of PCM-SABRE</blockquote> | |||
|- | |||
|} | |||
|} | |||
We designed PCM-SABRE workflow according to the common steps of knowledge discovery in data. First, the user can use a supplied dataset or load a new dataset. The dataset has to be a .csv file, in the form of a table in which the rows represent the patients and the columns represent clinical data, gene expression data, or any other types of variables. The dependent variable can be binary or continuous (it will be transformed into a binary variable) and needs to represent survival time (for example, relapse-free-survival time or death time). | |||
The second Meta-node is the preprocessing step, where a binary dependent variable is created and patients with missing data or censored survival information are being filtered. We chose to use a default threshold of five years in order to split the continues survival variable into HIGH (t < 5 years) or LOW (t ≤ 5 years) risk, but this threshold is an input parameter that can be changed in a way that will be explained later. Missing values imputation is performed using random forest classification that builds a model using the non-missing rows and predicts the variable value for the missing rows. The default version of PCM-SABRE allows selecting patients according to their ER status and lymph node status, but the "Select Patients" Meta-node is optional and can be easily modified to meet other inclusion/exclusion criteria. The third Meta-node is the feature selection step, where the users can choose between two methods of feature selection (information gain or ANOVA) or add another feature selection method (from the available nodes in KNIME, using scripting or external tools). The fourth Meta-node is the modeling step, where we offer a choice of five well-known and relevant classifiers. The methods included in the out-of-the-box basic version of the workflow are described in Table 1. | |||
{| | |||
| STYLE="vertical-align:top;"| | |||
{| class="wikitable" border="1" cellpadding="5" cellspacing="0" width="70%" | |||
|- | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" colspan="5"|'''Table 1.''' Machine learning methods available in PCM-SABRE | |||
|- | |||
! style="padding-left:10px; padding-right:10px;" colspan="2"|Meta-node | |||
! style="padding-left:10px; padding-right:10px;"|Method | |||
! style="padding-left:10px; padding-right:10px;"|KNIME node | |||
! style="padding-left:10px; padding-right:10px;"|Default parameters | |||
|- | |||
| style="background-color:white; padding-left:10px; padding-right:10px;"|1.1 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;"|Select Patients | |||
| style="background-color:white; padding-left:10px; padding-right:10px;"|Estrogen Receptor (ER) status | |||
| style="background-color:white; padding-left:10px; padding-right:10px;"|R script | |||
| style="background-color:white; padding-left:10px; padding-right:10px;"| | |||
|- | |||
| style="background-color:white; padding-left:10px; padding-right:10px;"|1.2 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;"|Select Patients | |||
| style="background-color:white; padding-left:10px; padding-right:10px;"|Lymph Node (LN) status | |||
| style="background-color:white; padding-left:10px; padding-right:10px;"|R script | |||
| style="background-color:white; padding-left:10px; padding-right:10px;"| | |||
|- | |||
| style="background-color:white; padding-left:10px; padding-right:10px;"|2.1 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;"|Feature Selection | |||
| style="background-color:white; padding-left:10px; padding-right:10px;"|Information Gain (InfoGain) | |||
| style="background-color:white; padding-left:10px; padding-right:10px;"|InformationGainCalculator (Community node – Palladian) | |||
| style="background-color:white; padding-left:10px; padding-right:10px;"|Top 100 ranked | |||
|- | |||
| style="background-color:white; padding-left:10px; padding-right:10px;"|2.2 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;"|Feature Selection | |||
| style="background-color:white; padding-left:10px; padding-right:10px;"|ANOVA | |||
| style="background-color:white; padding-left:10px; padding-right:10px;"|One-way ANOVA | |||
| style="background-color:white; padding-left:10px; padding-right:10px;"|Include genes with ''p''-value < 1.0E-6 | |||
|- | |||
| style="background-color:white; padding-left:10px; padding-right:10px;"|3.1 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;"|Modeling | |||
| style="background-color:white; padding-left:10px; padding-right:10px;"|Logistic Regression (LR) | |||
| style="background-color:white; padding-left:10px; padding-right:10px;"|Logistic (3.7) (Weka node) | |||
| style="background-color:white; padding-left:10px; padding-right:10px;"|Ridge = 1.0E-8, | |||
|- | |||
| style="background-color:white; padding-left:10px; padding-right:10px;"|3.2 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;"|Modeling | |||
| style="background-color:white; padding-left:10px; padding-right:10px;"|Random Forest (RF) | |||
| style="background-color:white; padding-left:10px; padding-right:10px;"|Random Forest Learner | |||
| style="background-color:white; padding-left:10px; padding-right:10px;"|Split criteria = Information Gain Ratio, Number of models = 350 | |||
|- | |||
| style="background-color:white; padding-left:10px; padding-right:10px;"|3.3 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;"|Modeling | |||
| style="background-color:white; padding-left:10px; padding-right:10px;"|Artificial Neural Network (ANN) | |||
| style="background-color:white; padding-left:10px; padding-right:10px;"|PNN Learner (DDA) | |||
| style="background-color:white; padding-left:10px; padding-right:10px;"|Theta Minus = 0.2, Theta Plus = 0.4 | |||
|- | |||
| style="background-color:white; padding-left:10px; padding-right:10px;"|3.4 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;"|Modeling | |||
| style="background-color:white; padding-left:10px; padding-right:10px;"|K-Nearest Neighbors (KNN) | |||
| style="background-color:white; padding-left:10px; padding-right:10px;"|IBK (3.7) (Weka node) | |||
| style="background-color:white; padding-left:10px; padding-right:10px;"|KNN = 15 | |||
|- | |||
| style="background-color:white; padding-left:10px; padding-right:10px;"|3.5 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;"|Modeling | |||
| style="background-color:white; padding-left:10px; padding-right:10px;"|Support Vector Machine (SVM) | |||
| style="background-color:white; padding-left:10px; padding-right:10px;"|SVM Learner | |||
| style="background-color:white; padding-left:10px; padding-right:10px;"|Kernel = RBF, sigma = 0.2 | |||
|- | |||
|} | |||
|} | |||
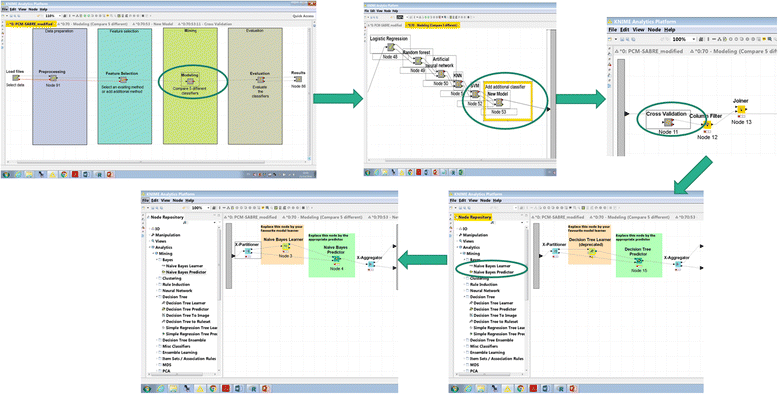
It should be noted that thanks to the design of KNIME, adding additional Modeling and Feature Selection methods involves just dropping additional nodes in the appropriate Meta-nodes and connecting them by drag-and-drop using the existing methods as templates. Our experience with experimental biologists suggests that any oncology researcher without programming capabilities can achieve this with little or no special training. Fig. 2 illustrates how the user can easily and quickly add additional classifier to the workflow: (1) double-click modeling → new model → cross-validation (2) delete the decision tree learner and predictor (3) choose from the Node Repository another learner and predictor nodes, then drag-and-drop them instead of the deleted nodes (4) connect the X-Partitioner node Training data output into the Learner node input, connect the Learner node PMML output into the PMML input of the Predictor node, connect the Predictor node to the X-Aggregator node, and connect the X-partitioner Test data output to the Predictor node. | |||
[[File:Fig2 Eyal-Altman BMCBioinformatics2017 18.gif|778px]] | |||
{{clear}} | |||
{| | |||
| STYLE="vertical-align:top;"| | |||
{| border="0" cellpadding="5" cellspacing="0" width="778px" | |||
|- | |||
| style="background-color:white; padding-left:10px; padding-right:10px;"| <blockquote>'''Figure 2.''' Demonstration of drag-and-drop model replacement (Naïve Bayes instead of decision tree)</blockquote> | |||
|- | |||
|} | |||
|} | |||
The fifth Meta-node is the evaluation step, which calculates the performance measures of different models (among them the accuracy and the area under the ROC). An important feature of PCM-SABRE is a .csv file (flow_variables.csv) that allows the user to control some default input parameters without the need to change these parameters inside the specific KNIME nodes. The controlled input parameters are: (1) Feature selection method (default = infoGain), ER status (default = all patients), Lymph node status (default = all patients) and the threshold for the binary survival variable (default = five years). Changing and adding another input parameter is simple and only requires filling cells in Excel. Additional details on how to use PCM-SABRE can be found in the user manual. | |||
Revision as of 19:46, 27 March 2017
| Full article title |
PCM-SABRE: A platform for benchmarking and comparing outcome prediction methods in precision cancer medicine |
|---|---|
| Journal | BMC Bioinformatics |
| Author(s) | Eyal-Altman, Noah; Last, Mark; Rubin, Eitan |
| Author affiliation(s) | Ben-Gurion University of the Negev |
| Primary contact | Email: eyalnoa at post dot bgu dor ac dot il |
| Year published | 2017 |
| Volume and issue | 18 |
| Page(s) | 40 |
| DOI | 10.1186/s12859-016-1435-5 |
| ISSN | 1471-2105 |
| Distribution license | Creative Commons Attribution 4.0 International |
| Website | http://bmcbioinformatics.biomedcentral.com/articles/10.1186/s12859-016-1435-5 |
| Download | http://bmcbioinformatics.biomedcentral.com/track/pdf/10.1186/s12859-016-1435-5 (PDF) |
|
|
This article should not be considered complete until this message box has been removed. This is a work in progress. |
Abstract
Background: Numerous publications attempt to predict cancer survival outcome from gene expression data using machine-learning methods. A direct comparison of these works is challenging for the following reasons: (1) inconsistent measures used to evaluate the performance of different models, and (2) incomplete specification of critical stages in the process of knowledge discovery. There is a need for a platform that would allow researchers to replicate previous works and to test the impact of changes in the knowledge discovery process on the accuracy of the induced models.
Results: We developed the PCM-SABRE platform, which supports the entire knowledge discovery process for cancer outcome analysis. PCM-SABRE was developed using KNIME. By using PCM-SABRE to reproduce the results of previously published works on breast cancer survival, we define a baseline for evaluating future attempts to predict cancer outcome with machine learning. We used PCM-SABRE to replicate previous work that describes predictive models of breast cancer recurrence, and tested the performance of all possible combinations of feature selection methods and data mining algorithms that was used in either of the works. We reconstructed the work of Chou et al. observing similar trends – superior performance of Probabilistic Neural Network (PNN) and logistic regression (LR) algorithms and inconclusive impact of feature pre-selection with the decision tree algorithm on subsequent analysis.
Conclusions: PCM-SABRE is a software tool that provides an intuitive environment for rapid development of predictive models in cancer precision medicine.
Keywords: Breast cancer, data mining, reproducible research
Background
Predicting the outcome of cancer from gene expression data is a clinically important, computationally challenging task. For example, early-stage, estrogen-receptor-positive, HER2-negative breast cancer patients that are considered to be at low risk for recurrence can avoid chemotherapy, while patients at high or intermediate risk are treated with aggressive (and harmful) chemotherapy.[1]
Efforts to stratify patients by risk of recurrence in other tumor types, and the ability to stratify patients by overall chances of survival are not as advanced. Moreover, the relative success in risk stratification for breast cancer patients has been challenged[2], proposing that it in fact stratifies patients into tumor subtypes, which can be achieved with much simpler tests.
As a result, a large number of papers have been published and are still being published where gene expression data is analyzed in order to construct models that predict cancer survival or cancer recurrence. Much of these efforts are concentrated on breast cancer, the second most commonly diagnosed cancer among American women (besides skin cancer).[3] About 1 in 8 U.S. women (about 12 percent) will develop invasive breast cancer over the course of her lifetime, and similar rates are reported worldwide.[4] Breast cancer is an attractive domain for risk stratification as it is estimated that resection is a sufficient treatment for 70 to 80 percent of the patients, while the remaining patients will develop advanced metastatic lesions, which are largely impossible to cure.[5] Aggressive chemotherapy will reduce the chance of advanced metastasis for those patients in that situation, though it would be harmful and unnecessary therapy for those who aren't. Thus, great efforts have been invested in stratifying patients’ risk of recurrence.[6]
Due to the importance of risk stratification in breast cancer, combined with its relatively high abundance, breast cancer is the type of tumor for which expression profiles of newly diagnosed patients are most abundant. Several works have been published that apply machine-learning techniques to this data for predicting cancer survivability.[7][8] Unfortunately, we found it quite challenging to directly compare these works for the following reasons:
- Incomplete specification of critical stages in the process of knowledge discovery, such as feature selection.
- Differences in the measures used to evaluate models performance. Some only provide the overall accuracy of the proposed classifier, some offer only the area under a curve (AUC), while others provide no statistical measures and only present the Kaplan-Meier charts that visualize the survival curves based on predicted classes.
- Different studies apply different inclusion/exclusion criteria with little or no overlaps between the patients considered.
Incomplete documentation of the analytic process is a common cause for irreproducibility of published results. We conclude that there is a need for a platform that would allow researchers to describe their analytic work in the field of risk stratification for cancer patients in a reproducible way that can be used for further investigation. Such a platform should allow the replication of previous works and methodologically evaluate the impact of alterations in one or more stages of the knowledge discovery process on its performance in the task of cancer survival prediction. Such a tool can help to understand and compare the current state of predictions for breast cancer, and if applied to new cancer types, to prevent the "Tower of Babel" situation that has emerged for breast cancer.
Implementation
We developed a platform that allows replicating, comparing and improving knowledge discovery pipelines for cancer survival predictions, and demonstrate its applicability for Breast Cancer (Fig. 1). PCM-SABRE (Precision Cancer Medicine - Survival Analysis Benchmarking, Reporting and Evaluation), was developed using KNIME (Konstanz Information Miner).[9] KNIME is a modern, flexible and intuitive open-source data analytics platform that allows performing sophisticated statistics and data mining analysis to develop, among other things, predictive models. We chose KNIME since it is a popular, user-friendly software that does not require programing knowledge. Its node-based workflow structure allows easily assessing the impact of changing one knowledge discovery step (for example, data mining algorithm) on the predictive performance without changing any other steps of the workflow. Another major benefit of KNIME is the ability to create new nodes; this feature is particularly useful when a researcher is interested in integrating a new method he developed into an existing KNIME workflow.
|
We designed PCM-SABRE workflow according to the common steps of knowledge discovery in data. First, the user can use a supplied dataset or load a new dataset. The dataset has to be a .csv file, in the form of a table in which the rows represent the patients and the columns represent clinical data, gene expression data, or any other types of variables. The dependent variable can be binary or continuous (it will be transformed into a binary variable) and needs to represent survival time (for example, relapse-free-survival time or death time).
The second Meta-node is the preprocessing step, where a binary dependent variable is created and patients with missing data or censored survival information are being filtered. We chose to use a default threshold of five years in order to split the continues survival variable into HIGH (t < 5 years) or LOW (t ≤ 5 years) risk, but this threshold is an input parameter that can be changed in a way that will be explained later. Missing values imputation is performed using random forest classification that builds a model using the non-missing rows and predicts the variable value for the missing rows. The default version of PCM-SABRE allows selecting patients according to their ER status and lymph node status, but the "Select Patients" Meta-node is optional and can be easily modified to meet other inclusion/exclusion criteria. The third Meta-node is the feature selection step, where the users can choose between two methods of feature selection (information gain or ANOVA) or add another feature selection method (from the available nodes in KNIME, using scripting or external tools). The fourth Meta-node is the modeling step, where we offer a choice of five well-known and relevant classifiers. The methods included in the out-of-the-box basic version of the workflow are described in Table 1.
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||
It should be noted that thanks to the design of KNIME, adding additional Modeling and Feature Selection methods involves just dropping additional nodes in the appropriate Meta-nodes and connecting them by drag-and-drop using the existing methods as templates. Our experience with experimental biologists suggests that any oncology researcher without programming capabilities can achieve this with little or no special training. Fig. 2 illustrates how the user can easily and quickly add additional classifier to the workflow: (1) double-click modeling → new model → cross-validation (2) delete the decision tree learner and predictor (3) choose from the Node Repository another learner and predictor nodes, then drag-and-drop them instead of the deleted nodes (4) connect the X-Partitioner node Training data output into the Learner node input, connect the Learner node PMML output into the PMML input of the Predictor node, connect the Predictor node to the X-Aggregator node, and connect the X-partitioner Test data output to the Predictor node.
|
The fifth Meta-node is the evaluation step, which calculates the performance measures of different models (among them the accuracy and the area under the ROC). An important feature of PCM-SABRE is a .csv file (flow_variables.csv) that allows the user to control some default input parameters without the need to change these parameters inside the specific KNIME nodes. The controlled input parameters are: (1) Feature selection method (default = infoGain), ER status (default = all patients), Lymph node status (default = all patients) and the threshold for the binary survival variable (default = five years). Changing and adding another input parameter is simple and only requires filling cells in Excel. Additional details on how to use PCM-SABRE can be found in the user manual.
References
- ↑ Sparano, J.A.; Gray, R.J.; Makower, D.F. et al. (2015). "Prospective Validation of a 21-Gene Expression Assay in Breast Cancer". New England Journal of Medicine 373 (21): 2005–14. doi:10.1056/NEJMoa1510764. PMID 26412349.
- ↑ Senkus, E.; Kyriakides, S.; Ohno, S. et al. (2015). "Primary breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up". Annals of Oncology 26 (Suppl 5): v8-30. doi:10.1093/annonc/mdv298. PMID 26314782.
- ↑ "U.S. Breast Cancer Statistics". Breastcancer.org. 2016. http://www.breastcancer.org/symptoms/understand_bc/statistics. Retrieved 20 December 2016.
- ↑ "Breast cancer statistics". World Cancer Research Fund International. 2016. http://www.wcrf.org/int/cancer-facts-figures/data-specific-cancers/breast-cancer-statistics. Retrieved 20 December 2016.
- ↑ "Statistics for Metastatic Breast Cancer". Metastatic Breast Cancer Network. 2016. http://www.mbcn.org/statistics-for-metastatic-breast-cancer/. Retrieved 20 December 2016.
- ↑ Cronin, M.; Sangli, C.; Liu, M.L. et al. (2007). "Analytical validation of the Oncotype DX genomic diagnostic test for recurrence prognosis and therapeutic response prediction in node-negative, estrogen receptor-positive breast cancer". Clinical Chemistry 53 (6): 1084-91. doi:10.1373/clinchem.2006.076497. PMID 17463177.
- ↑ Györffy, B.; Lanczky, A.; Eklund, A.C. et al. (2010). "An online survival analysis tool to rapidly assess the effect of 22,277 genes on breast cancer prognosis using microarray data of 1,809 patients". Breast Cancer Research and Treatment 123 (3): 725-31. doi:10.1007/s10549-009-0674-9. PMID 20020197.
- ↑ Naoi, Y.; Kishi, K.; Tanei, T. et al. (2011). "Development of 95-gene classifier as a powerful predictor of recurrences in node-negative and ER-positive breast cancer patients". Breast Cancer Research and Treatment 128 (3): 633-41. doi:10.1007/s10549-010-1145-z. PMID 20803240.
- ↑ Berthold, M.R.; Cebron, N.; Dill, F. et al. (2008). "KNIME: The Konstanz Information Miner". In Preisach, C.; Burkhardt, H.; Schmidt-Thieme, L.; Decker, R. (PDF). Data Analysis, Machine Learning and Applications. Springer-Verlag Berlin Heidelberg. pp. 319–326. doi:10.1007/978-3-540-78246-9. ISBN 9783540782469. http://www.inf.uni-konstanz.de/bioml2/publications/Papers2007/BCDG+07_knime_gfkl.pdf.
Notes
This presentation is faithful to the original, with only a few minor changes to presentation. In some cases important information was missing from the references, and that information was added. Some grammar were corrected when necessary.