Difference between revisions of "ELN feature"
Shawndouglas (talk | contribs) m (→Data and trend analysis: Grammar.) |
(Reverting to last revision not containing links to *.ch) |
||
| Line 27: | Line 27: | ||
<div class="mw-collapsible" style="width:100%; background-color:white;"> | <div class="mw-collapsible" style="width:100%; background-color:white;"> | ||
<p> </p> | <p> </p> | ||
===Chemical | ===Chemical drawing and calculation=== | ||
===Chemical and/or spectrum file support=== | |||
===Reagents database=== | |||
===Task and event scheduling=== | ===Task and event scheduling=== | ||
| Line 64: | Line 59: | ||
The ELN is particularly useful for displaying chemical structures and bonds in more than one way through built-in or integratable chemical structure drawing tools or through a document manager that can handle the placement of screenshots, sketches, or other images. | The ELN is particularly useful for displaying chemical structures and bonds in more than one way through built-in or integratable chemical structure drawing tools or through a document manager that can handle the placement of screenshots, sketches, or other images. | ||
===Data and trend analysis=== | ===Data and trend analysis=== | ||
| Line 73: | Line 64: | ||
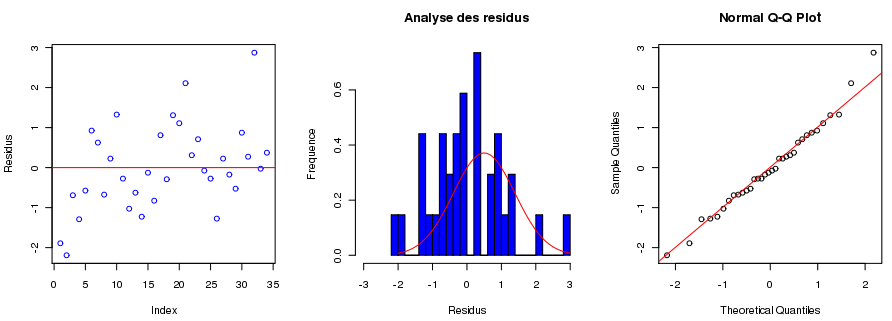
[[File:Analyse residus.png|thumb|260px|left|Some ELNs allow users to analyze experiment data with built-in software tools.]] Sample experimentation and [[Data analysis|analysis]] plays an important part of laboratory informatics, helping laboratories make better sense of their experiments and reach valuable conclusions about them. While this important phase of laboratory work has often been done externally from the ELN, it's now more common to see basic analysis tools being included. Such tools allow raw data to be imported directly to the ELN, which then can store, process, and display it in a shareable form. Additionally, chemical calculations and functions used in the analysis are typically definable and editable for further flexibility. | [[File:Analyse residus.png|thumb|260px|left|Some ELNs allow users to analyze experiment data with built-in software tools.]] Sample experimentation and [[Data analysis|analysis]] plays an important part of laboratory informatics, helping laboratories make better sense of their experiments and reach valuable conclusions about them. While this important phase of laboratory work has often been done externally from the ELN, it's now more common to see basic analysis tools being included. Such tools allow raw data to be imported directly to the ELN, which then can store, process, and display it in a shareable form. Additionally, chemical calculations and functions used in the analysis are typically definable and editable for further flexibility. | ||
As with the feature "multiple data viewing methods," data and trend analysis is also increasingly important in laboratories that have very specialized data management needs. When even in 2009 genetic scientists in large- and medium-sized [[sequencing]] and core centers were voicing concerns about "a lack of adequate vendor-supported software and laboratory information management systems (LIMS),"<ref name=PLoS0506>{{cite journal|journal=PLoS Computational Biology |year=2009 |volume=5 |issue=6 |title=Managing and Analyzing Next-Generation Sequence Data |author=Richter, Brent G.; David P. Sexton |url=http://www.ploscompbiol.org/article/info%3Adoi%2F10.1371%2Fjournal.pcbi.1000369 |doi=10.1371/journal.pcbi.1000369}}</ref>, today data management options like | As with the feature "multiple data viewing methods," data and trend analysis is also increasingly important in laboratories that have very specialized data management needs. When even in 2009 genetic scientists in large- and medium-sized [[sequencing]] and core centers were voicing concerns about "a lack of adequate vendor-supported software and laboratory information management systems (LIMS),"<ref name=PLoS0506>{{cite journal|journal=PLoS Computational Biology |year=2009 |volume=5 |issue=6 |title=Managing and Analyzing Next-Generation Sequence Data |author=Richter, Brent G.; David P. Sexton |url=http://www.ploscompbiol.org/article/info%3Adoi%2F10.1371%2Fjournal.pcbi.1000369 |doi=10.1371/journal.pcbi.1000369}}</ref>, today data management options like [[Scilligence Corporation#Scilligence ELN|Scilligence ELN]] are starting to emerge, offering the ability to perform specialized analytical tasks for the researcher.<ref name="ScillELNHome">{{cite web |url=http://www.scilligence.com/web/eln.aspx |title=Scilligence ELN |publisher=Scilligence Corporation |accessdate=07 February 2013}}</ref> | ||
As sample experimentation and data analysis are important parts of most if not all laboratories, such functionality — which has often come in the form of a separate application or analysis device — will likely continue to merge into software like ELN, LIMS, and other [[laboratory informatics]] solutions.<ref name=AE0302>{{cite journal|journal=Automated Experimentation |year=2011 |volume=3 |issue=2 |title=The benefits of integrated systems for managing both samples and experimental data: An opportunity for labs in universities and government research institutions to lead the way |author=Macneil, Rory |doi=10.1186/1759-4499-3-2 |url=http://www.aejournal.net/content/3/1/2}}</ref> | As sample experimentation and data analysis are important parts of most if not all laboratories, such functionality — which has often come in the form of a separate application or analysis device — will likely continue to merge into software like ELN, LIMS, and other [[laboratory informatics]] solutions.<ref name=AE0302>{{cite journal|journal=Automated Experimentation |year=2011 |volume=3 |issue=2 |title=The benefits of integrated systems for managing both samples and experimental data: An opportunity for labs in universities and government research institutions to lead the way |author=Macneil, Rory |doi=10.1186/1759-4499-3-2 |url=http://www.aejournal.net/content/3/1/2}}</ref> | ||
| Line 85: | Line 76: | ||
As thorough as some user interface (UI) developers may be in adding relevant fields and interface options for eln end users, there are at times options that are either omitted or unanticipated. This has traditionally required the end user to contact the vendor and ask if the needed option(s) can be added in the next release. However, many modern ELN vendors have responded instead by adding functionality that gives end users and/or ELN administrators more control over the user interface. | As thorough as some user interface (UI) developers may be in adding relevant fields and interface options for eln end users, there are at times options that are either omitted or unanticipated. This has traditionally required the end user to contact the vendor and ask if the needed option(s) can be added in the next release. However, many modern ELN vendors have responded instead by adding functionality that gives end users and/or ELN administrators more control over the user interface. | ||
Aspects of the ELN's user interface that are often customizable by the end user include: | Aspects of the ELN's user interface that are often customizable by the end user include:<ref> | ||
* | * experiment templates and forms | ||
* | * | ||
===Query capability=== | ===Query capability=== | ||
[[File: | [[File:QueryBuilder.png|thumb|240px|Advanced query tools allow researchers to better complete project objectives.]]As was the case before the advent of databases and electronic data management solutions, today researchers must search through sample results, experiment notes, and other types of data to better draw conclusions about their research. Whereas this used to mean browsing through laboratory notebooks, Excel spreadsheets, or Access databases, now powerful query tools exists within data management tools like a LIMS. Not only can data be searched for based on name, number, or vendor, LIMSs like [[eBioSys Pty. Ltd.|eBioSys']] eLab and [[Mountain States Consulting, LLC|Mountain States Consulting's]] MSC-LIMS allow for queries of attached meta-data like user ID, project number, task number, sample type, location, and collection date.<ref>{{cite web_short|url=http://www.ebiosys.com/SampleManagement.aspx |title=Products - eLab - Sample Management |publisher=eBioSys Pty. Ltd. |accessdate=15 February 2012}}</ref><ref>{{cite web_short|url=http://www.msc-lims.com/lims/msc-lims.html |title=MSC-LIMS Product Summary |publisher=Mountain States Consulting, LLC |accessdate=15 February 2012}}</ref> Finally, as LIMS continue to include both sample management and experimental data management functionality, queries become more powerful in general as now sample and experiment can be matched together in one database.<ref name=AE0302 /> | ||
Query functionality often includes the ability to: | Query functionality often includes the ability to: | ||
* search both transactional data and archived data tables | * search both transactional data and archived data tables | ||
* search multiple databases via an application programming interface (API) or open database connectivity (ODBC) connection | * search multiple databases via an application programming interface (API) or open database connectivity (ODBC) connection | ||
* filter and sort data | * filter and sort data | ||
* create ad-hoc queries | * create ad-hoc queries | ||
===Import data=== | ===Import data=== | ||
Data can originate from numerous places in the laboratory. The ability to import that data into | Data can originate from numerous places in the laboratory. The ability to import that data into a LIMS can be beneficial, especially when an instrument can't be connected or an external client provides a data feed independent of the LIMS. Some LIMS like [[Bridge-Soft, LLC|Bridge-Soft's]] QMS even allow to cross-reference laboratory nomenclature from received data sources with the recipient's.<ref>{{cite web_short|url=http://www.bridge-soft.com/Products/Solutions.aspx |title=Water and Wastewater Solutions |publisher=Bridge-Soft, LLC |accessdate=17 February 2012}}</ref> And of course [[LIMS feature#Instrument interfacing and management|instrument interfacing]] allows for even more importation options. Additional [[LIMS_feature#Data validation|data validation]] procedures may be applied to the imported data to guarantee information homogeneity. Additionally, some LIMS may allow for the import and integration of non-normalized legacy data tables with LIMS data tables into a single database. | ||
===Internal file or data linking=== | ===Internal file or data linking=== | ||
This feature allows | This feature allows LIMS users to link together reports, protocols, sample results, and more, providing greater contextual clarity to projects. Examples include: | ||
* linking a sample batch to | * linking a sample batch to a test or sample preparation methodology | ||
* linking a test process to a particular | * linking a test process to a particular customer | ||
* linking a report to a sample batch | * linking a report to a sample batch | ||
* linking a group of | * linking a group of test results to a raw data file | ||
* linking | * linking an image to a work order | ||
* linking all | * linking all lab results with the correct reporting test method | ||
===External file or data linking=== | ===External file or data linking=== | ||
This feature allows | This feature allows LIMS users to link together data and files in the LIMS with data, files, and customers outside the scope of the LIMS. Examples include: | ||
* linking to | * linking data files from chromatography equipment to synthesis data<ref>{{cite web_short|url=http://www.2sslab.com/Features.html |title=Get OhNo! for Radiopharmaceutical Control |publisher=2nd Sight Solutions |accessdate=17 February 2012}}</ref> | ||
* linking external | * linking equipment ID with an external annotation database<ref>{{cite web_short|url=http://www.integromics.com/genomics-data-analysis/pcr-analysis/datasheet |title=RealTime StatMiner for Real-time qPCR Data Analysis |format=PDF |publisher=Integromics SL |accessdate=17 February 2012}}</ref> | ||
* linking external standard operating procedure documents with an internal test specification<ref>{{cite web_short|url=http://www.labware.com/LWWeb.nsf/lp/en040108 |title=LabWare External Links and Document Management |publisher=LabWare, Inc. |accessdate=17 February 2012}}</ref> | |||
===ELN support or integration=== | |||
As a software replacement for more traditional paper laboratory notebooks, the [[electronic laboratory notebook]] (ELN) has been important to laboratory functions. Yet the lines between ELN and LIMS began to blur in the 2000s, with both types of software incorporating features from the other.<ref name="SciCompWorld1">{{cite web|url=http://www.scientific-computing.com/features/feature.php?feature_id=50|author=Elliot, Michael H.|title=The state of the ELN Market |publisher=Scientific Computing World |date=December 2006–January 2007 |accessdate=04 May 2011}}</ref><ref name="SciCompELN">{{cite web|url=http://www.scientificcomputing.com/articles-IN-Informatics-Convergence-Presents-Opportunities-and-Challenges-111111.aspx |author=Elliot, Michael H. |title=Informatics Convergence Presents Opportunities and Challenges |publisher=Scientific Computing |date=October 2011 |accessdate=25 February 2012}}</ref> The result today is some LIMS either include traditional ELN functionality or link physical laboratory notebook references to data in the LIMS.<ref>{{cite web_short|url=http://www.labware.com/LWWeb.nsf/lp/en040109 |title=LabWare Electronic Lab Notebooks |publisher=LabWare, Inc. |accessdate=17 February 2012}}</ref> | |||
===Export to MS Excel=== | ===Export to MS Excel=== | ||
While Microsoft Excel has long been used within the laboratory setting, a slow shift towards relational databases and LIMS occurred in the late 1990s and early 2000s.<ref name=WilliamsExcel>{{cite web|url=http://www.sfn.org/skins/main/pdf/ShortCourses/2003/sc1_9.pdf |author=Williams, Robert W. |title=Managing Your Lab Data Flux: Getting Beyond Excel |work=The Bioinformatics of Brains: From Genes and Proteins to Behaviors |publisher=Washington, DC: Society for Neuroscience |format=PDF |date=2003 |accessdate=17 February 2012}}</ref> Additional concerns with the difficulties of Excel's validation and compliance with FDA [[21 CFR Part 11]] and other regulations have led many labs to turn to data management solutions that are easier to validate.<ref name=PragmaticExcelIntro>{{cite journal|journal=Pharma IT |year=2007 |volume=1 |issue=2 |pages=30–35 |title=A Pragmatic Approach to the Validation of Excel Spreadsheets – Overview |author=Howard, David A.; David Harrison |format=PDF |url=http://www.spreadsheetvalidation.com/pdf/Excel_Spreadsheet_Validation_Overview.pdf}}</ref> Nevertheless, laboratories continue to use Excel in some fashion, and thus Excel integration or data exportation in Excel format is a real need for | While Microsoft Excel has long been used within the laboratory setting, a slow shift towards relational databases and LIMS occurred in the late 1990s and early 2000s.<ref name=WilliamsExcel>{{cite web|url=http://www.sfn.org/skins/main/pdf/ShortCourses/2003/sc1_9.pdf |author=Williams, Robert W. |title=Managing Your Lab Data Flux: Getting Beyond Excel |work=The Bioinformatics of Brains: From Genes and Proteins to Behaviors |publisher=Washington, DC: Society for Neuroscience |format=PDF |date=2003 |accessdate=17 February 2012}}</ref> Additional concerns with the difficulties of Excel's validation and compliance with FDA [[21 CFR Part 11]] and other regulations have led many labs to turn to data management solutions that are easier to validate.<ref name=PragmaticExcelIntro>{{cite journal|journal=Pharma IT |year=2007 |volume=1 |issue=2 |pages=30–35 |title=A Pragmatic Approach to the Validation of Excel Spreadsheets – Overview |author=Howard, David A.; David Harrison |format=PDF |url=http://www.spreadsheetvalidation.com/pdf/Excel_Spreadsheet_Validation_Overview.pdf}}</ref> Nevertheless, laboratories continue to use Excel in some fashion, and thus Excel integration or data exportation in Excel format is a real need for LIMS customers. LIMS with this feature allow raw, processed, or imported data to be exported in the Excel format for further analysis and dissemination. | ||
===Raw data management=== | ===Raw data management=== | ||
While not described as a feature on most | While not described as a feature on most LIMS vendor websites, a few indicate that their LIMS is capable of managing (import, export, editing, etc.) data in its raw format for future analysis. | ||
===Data warehouse=== | ===Data warehouse=== | ||
A LIMS' [[data warehouse]] serves the important function of storing, extracting, and managing the data that laboratories crank out for the purposes or analysis, reporting, and process management, typically separate from the primary storage database. Data warehouses also offer the benefit of speeding up queries, making queries and data mining more user-friendly, and smoothing out data gaps.<ref>{{cite web |url=http://www.labvantage.com/blog/?p=79 |title=Advantages of a Data Warehouse |author=Vannest, Jeff |publisher=LABVANTAGE Solutions, Inc. |date=11 February 2011 |accessdate=17 February 2012}}</ref> | |||
===Deadline control=== | |||
Deadline control is functionality within a LIMS that allows users to manage and be notified of events that occur within the laboratory. With this functionality users can also be notified of upcoming deadlines on anything from sample analysis to license renewal. | |||
Note that this functionality may also feasibly fall under the [[LIMS feature#Task and event scheduling|task and event scheduling]] or [[LIMS feature#Alarms and/or alerts|alarms]] features. As deadline control seems to be advertised as a notable feature by only a few vendors, it seems even more likely that this functionality is considered part of scheduling or alarms. | |||
===Production control=== | |||
There are many types of businesses that produce goods, and in most cases there is a research laboratory involved at some point in the process. This is especially true in the pharmaceutical and chemical industries, where production measurements such as yield, volume, activity, and impurity are vital. As LIMSs have already recorded such information during tests and analysis, the addition of production control functionality seems natural. Some LIMS take a very active approach to this. For example, [[2nd Sight Solutions|2nd Sight Solutions']] OhNo! features production control as major functionality for the synthesis of [[Radiopharmacology|radiopharmaceuticals]].<ref>{{cite web_short|url=http://www.2sslab.com/Production.html |title=Use OhNo! for complete control |publisher=2nd Sight Solutions |accessdate=20 February 2012}}</ref> Other LIMS may have less pronounced production functionality, while still offering the ability to track the production process in and out of the lab. And yet other LIMSs like [[dialog EDV Systementwicklung GmbH|dialog's]] diaLIMS offer robust production-based functionality but as a module or add-on to the base LIMS software.<ref>{{cite web_short|url=http://www.dialims.de/standardloesungen/lims-produktionsunterstuetzung.html |title=Standardlösung für die Produktion |publisher=dialog EDV Systementwicklung GmbH |accessdate=20 February 2012}}</ref> | |||
The types of functionality that may fall under this feature include: | |||
* recipe management | |||
* consumable tracking | |||
* batch traceability | |||
* production planning | |||
* enterprise resource planning | |||
===Project and task management=== | ===Project and/or task management=== | ||
Project and task management within | Project and task management within a LIMS typically involves the scheduling of tasks to workers and organizing associated tasks into a more cohesive unit for better tracking and management. While the functionality of [[LIMS_feature#Task and event scheduling|task and event scheduling]] can also be found in project and task management, many LIMS include functionality beyond scheduling that warrants the addition of the project and/or task management feature. This functionality includes: | ||
* job allocation and rescheduling | * job allocation and rescheduling | ||
* instrument workload tracking | * instrument workload tracking | ||
* [[LIMS_feature#Work-related time tracking|time tracking]] | |||
* pending workload verification | * pending workload verification | ||
* project | * priority setting | ||
* sample, batch, and document [[ | * project-based [[LIMS_feature#Workflow management|workflow management]] | ||
* work | * sample, batch, and document [[LIMS feature#Internal file or data linking|linking]] | ||
* work list sharing | |||
* recurring event management | * recurring event management | ||
===Inventory management=== | ===Inventory management=== | ||
[[File: | [[File:Laboratory-reagents.jpg|thumb|240px|LIMS can help laboratories keep track of their stock of reagents and even streamline reordering of them.]]Laboratories use a wide array of inventory, from [[Reagent|reagents]] to glassware, from [[Radiopharmacology|radiopharmaceuticals]] to [[Laboratory bath|laboratory baths]]. With that comes the need to know how much/many and the frequency of use. For this, most LIMS products now include some sort of inventory management functionality. | ||
* register | * register origin, demographics of incoming materials | ||
* track used and in-use items via | * track used and in-use items via barcodes | ||
* track inventory reduction based on usage and shipping out of the lab | * track inventory reduction based on usage and shipping out of the lab | ||
* create alerts for when items reach a certain stock level | * create alerts for when items reach a certain stock level | ||
| Line 164: | Line 175: | ||
* manual incrementing/decrementing of items | * manual incrementing/decrementing of items | ||
* track location and usage of laboratory equipment | * track location and usage of laboratory equipment | ||
* assign storage locations | * assign storage locations | ||
* track forensic evidence | * track forensic evidence | ||
It should be noted electronic equipment may also be considered inventory, and thus there is likely some functionality crossover with [[ | It should be noted that samples and electronic equipment may also be considered inventory, and thus there is likely some functionality crossover with the [[LIMS feature#Sample login and management|sample management]] and [[LIMS feature#Instrument interfacing and management|instrument management]] features. | ||
===Document creation and management=== | ===Document creation and management=== | ||
Standard operation procedures, (SOPs), specifications, reports, graphs, images, and receipts are all collected and used in the average laboratory. With | Standard operation procedures, (SOPs), specifications, reports, graphs, images, and receipts are all collected and used in the average laboratory. With a LIMS already designed to manage and store sample and experiment data, it makes sense to include functionality to create, import, export, and manage other sorts of data files. As sample and experimental data can be indexed, queried, and linked, so too can document data. Functionality of a typical document management system includes the ability to: | ||
* upload and index documents | * upload and index documents | ||
* enforce [[LIMS feature#Version control|version control]] | |||
* enforce [[ | * provide full text search | ||
* provide full text | * export to PDF or other relevant format | ||
* export to PDF | |||
* add documents as attachments | * add documents as attachments | ||
=== | ===Case management=== | ||
The [[laboratory information system]] (LIS) has played an important role in the case management tasks of patient-centric and clinical laboratories. However, some LIMS have gained case management functionality, effectively blurring the lines between LIS and LIMS.<ref>{{cite web|url=http://blog.starlims.com/2009/07/01/swimming-in-the-clinical-pool-why-lims-are-supplanting-old-school-clinical-lis-applications/|title=Swimming in the Clinical Pool: Why LIMS are supplanting old-school clinical LIS applications|author=Hice, Randy |publisher=STARLIMS' Laboratory Informatics Blog|date=01 July 2009|accessdate=09 May 2011}}</ref>. Self-proclaimed LIMS products have emerged in the clinical, public health, and veterinary industries, areas that have historically been served by LIS software. When also considering the fields of law enforcement and forensic science, case management has an increasingly important role in some LIMS. Functionality seen in the case management feature includes: | |||
* case accessioning and assignment | |||
* disease tracking | |||
* trend analysis | |||
* clinical history follow-up | |||
* out-of-range result [[LIMS feature#Alarms and/or alerts|alerts]] | |||
* document and result association | |||
* evidence control | |||
* study management | |||
=== | ===Workflow management=== | ||
[[File:Workflow IA.png|left|thumb|Capturing workflow in the lab is becoming more commonplace for the LIMS.]][[Workflow]] management is common in the laboratory, acting as a graphical representation of planned sequential steps to either fully or partially automate a process within the lab. Separate standards-based workflow management systems (in the form of a software component) have traditionally performed this task.<ref name=WfMC1>{{cite web |url=http://www.wfmc.org/standards/docs/TC-1011_term_glossary_v3.pdf |title=Workflow Management Coalition Terminology & Glossary |publisher=Workflow Management Coalition |pages=9 |format=PDF |date=February 1999 |accessdate=20 February 2012}}</ref> However, in the 2000s LIMS vendors began incorporating workflow management functionality into their LIMS software, reducing the headaches that customization of a LIMS often brought.<ref name=SciCompWF>{{cite web |url=http://www.scientificcomputing.com/using-workflows-in-lims-to-reduce.aspx |title=Using Workflows in LIMS to Reduce Customization |author=Maxwell, Glen |publisher=Scientific Computing and Instrumentation |date=1 November 2003 |accessdate=20 February 2012}}</ref> | |||
<br /> | |||
Modern commercial and open-source LIMS solutions often feature workflow management functionality, including<ref name=SIGLa>{{cite journal|journal=BMC Genomics |year=2010 |volume=11 |issue=Suppl 5 |pages=S8 |title=SIGLa: an adaptable LIMS for multiple laboratories |author=Melo, Alexandre; Alessandra Faria-Campos; Daiane M DeLaat; Rodrigo Keller; Vinícius Abreu; Sérgio Campos |url=http://www.biomedcentral.com/1471-2164/11/S5/S8 |doi=10.1186/1471-2164-11-S5-S8}}</ref><ref name=X-LIMS>{{cite web |url=http://www.ethosoft.com/LIMS/XLIMSProductInfo/XLIMSFAQ/tabid/69/Default.aspx |title=My laboratory has unique processes, Can X-LIMS work for me? |publisher=EthoSoft, Inc. |accessdate=20 February 2012}}</ref><ref name=SciCompWF />: | |||
=== | * attribute definition of activities | ||
* definition of inputs and outputs of activities | |||
* assignment of documentation to activities | |||
* setting of quality control limits | |||
* dynamically modify workflow in case of future changes | |||
* receive notification of changes to the workflow | |||
* sending user-defined messages during the process | |||
<br /> | |||
===Specification management=== | |||
[[ | [[Specification (technical standard)|Specification]] (spec) management is vital to not only the manufacturing and research industries but also to a host of other laboratories requiring precise measurements and infallible test methods. Just as the [[ASTM International|ASTM]] offers standards and specs for LIMS<ref name=ASTM_LIMS>{{cite web |url=http://www.astm.org/Standards/E1578.htm |title=ASTM E1578 - 06 Standard Guide for Laboratory Information Management Systems (LIMS) |publisher=ASTM International |accessdate=22 February 2012}}</ref>, so too do LIMS users have standards and specs for their laboratory. With spec management in place within the LIMS, laboratories can then: | ||
* enforce standard operating procedures and business rules | |||
* create specs down to a project or sample level | |||
* validate recipes and procedures | |||
* accept or reject sample batches | |||
* document internal and external spec history | |||
Note that some of the functionality of spec management may cross over into the realm of [[LIMS feature#QA/QC functions|quality control]] and [[LIMS feature#Data validation|data validation]]. | |||
===Customer and supplier management=== | ===Customer and supplier management=== | ||
Unless a laboratory is conducting internalized independent research, in most cases it will do business with external entities such as contract labs, sample providers, equipment providers, and reagent suppliers. In some cases, even internal employees may be considered a customer, necessitating documentation of who is using the system and in what ways. For a veterinary lab, the customer may be the animal and handler. For a forensic lab the customer may be more complex: internal staff, university staff, police departments, and maintainers of nationwide crime databases may all at some point act as customers. In these cases, documenting these various points of contact and linking them to | Unless a laboratory is conducting internalized independent research, in most cases it will do business with external entities such as contract labs, sample providers, equipment providers, and reagent suppliers. In some cases, even internal employees may be considered a customer, necessitating documentation of who is using the system and in what ways. For a veterinary lab, the customer may be the animal and handler. For a forensic lab the customer may be more complex: internal staff, university staff, police departments, and maintainers of nationwide crime databases may all at some point act as customers. In these cases, documenting these various points of contact and linking them to samples, equipment, and tests becomes vital. Managing demographics, complaints, correspondence, and history are all feasible with customer management functionality. This process is often made simpler through the use of a more context-neutral entity creation system, which allows for more flexible management of contacts. | ||
This feature may also be referred to as contact management, an address book module, or a customer service module. | This feature may also be referred to as contact management, an address book module, or a customer service module. | ||
===Billing management=== | |||
While the finances of a laboratory are important, they've typically been handled separately as a business process. However, some LIMS include additional functionality to make handling financial transactions and documentation of all sorts possible within the LIMS. In theory, such functionality brings the possibility of keeping more of a laboratory's data centrally located and queryable. This feature may include: | |||
* payment processing | |||
* expense reporting | |||
* price quotes | |||
* revenue management | |||
* workload tracking of billable hours | |||
* bill of materials | |||
* grant management | |||
</div> | </div> | ||
| Line 222: | Line 259: | ||
===Regulatory compliance=== | ===Regulatory compliance=== | ||
The topic of whether or not | The topic of whether or not a LIMS meets regulatory compliance is often a complex one. While Title [[21 CFR Part 11]] has arguably had the largest influence on a electronic data management system's compliance, other influential standards have shaped the way LIMS and other systems handle and store data. Other compliance-based codes, standards, and regulations include: | ||
* [[ASTM International|ASTM]] | * [[ASTM International|ASTM]] | ||
| Line 231: | Line 268: | ||
* [[Health Insurance Portability and Accountability Act|HIPAA]] | * [[Health Insurance Portability and Accountability Act|HIPAA]] | ||
* [[Health Level 7]] | * [[Health Level 7]] | ||
* [[ISO/IEC 17025]] | * [[ISO/IEC 17025]] | ||
* [[ISO 9000|ISO 9000/9001]] | * [[ISO 9000|ISO 9000/9001]] | ||
| Line 239: | Line 275: | ||
* Title [[40 CFR Part 3]] | * Title [[40 CFR Part 3]] | ||
With so many codes, standards, and regulations, | With so many codes, standards, and regulations, LIMS consumers are advised to contact vendors with their user requirements and ask how the vendor's software meets and/or exceeds those requirements. | ||
===QA/QC functions=== | ===QA/QC functions=== | ||
The quality management functions of | The quality management functions of a LIMS allow users to maintain a level of necessary quality across many of the functions in a laboratory. From running quality assurance tests to ensuring employed researchers are proficient at certain tasks, the QA/QC functionality of a LIMS is largely responsible for the output of consistent data and manufactured products in and out of the lab. | ||
Common functionality includes<ref name="AL3218">{{cite journal |journal=American Laboratory |year=2000 |volume=32 |issue=18 |pages=38–42 |title=Considerations in selecting a laboratory information management system (LIMS) |author=Paszko, Christine; Carol Pugsley |doi=10.1016/0169-7439(94)90006-X |url=http://www.mendeley.com/research/considerations-selecting-laboratory-information-management-system-lims/}}</ref><ref name="CCHTS1409">{{cite journal |journal=Combinatorial Chemistry & High Throughput Screening |year=2011 |volume=14 |issue=9 |pages=772–780 |title=Tracking and Controlling Everything that Affects Quality is the Key to a Quality Management System |author=Hull, Carl; Bruce Wray; Ford Winslow; Mark Vilicich |doi=10.2174/138620711796957125 |url=http://www.uniconnect.com/wp-content/uploads/2011/11/CCHTS-LIMS-Article.pdf |format=PDF}}</ref>: | |||
* single or batch QA/QC tests | |||
* quality control charts and reports | |||
* proficiency testing | |||
* [[LIMS feature#Document management|document management]] | |||
* instrument maintenance | |||
* data acceptance/rejection | |||
* certificates of analysis (COA) | |||
* data types defined by QC analysis | |||
===Performance evaluation=== | ===Performance evaluation=== | ||
As [[ | As [[LIMS feature#Document creation and management|document management]] becomes increasingly prevalent in LIMS, it only makes sense to also collate and store all the documentation associated with employee training and certification. Changes to laboratory techniques, scientific understanding, and business practices force researchers to learn, reevaluate, and demonstrate competency in order to maintain quality levels in the laboratory. Evaluations can frequently extend beyond staff members, however. Clinics, visit types, vendors, or test species can also be tracked and evaluated based on custom criteria. The performance evaluation functionality of a LIMS makes this possible. | ||
That functionality typically includes the ability to maintain training records and history, and also to link that training to a technique or piece of equipment. Afterwards, the staff member, vendor, etc. can be marked as competent or certified in the equipment, knowledge, or process. Periodical assessment of the training and its practical effectiveness can later be performed. Productivity of an entity or process can also be gauged over a certain date range based on [[ | That functionality typically includes the ability to maintain training records and history, and also to link that training to a technique or piece of equipment. Afterwards, the staff member, vendor, etc. can be marked as competent or certified in the equipment, knowledge, or process. Periodical assessment of the training and its practical effectiveness can later be performed. Productivity of an entity or process can also be gauged over a certain date range based on [[LIMS feature#Work-related time tracking|tracked time]], pre-determined milestones, or some other criteria. | ||
===Audit trail=== | ===Audit trail=== | ||
[[File:Syvalidate screen.jpg|thumb|left|Whether validating | [[File:Syvalidate screen.jpg|thumb|left|Whether validating sample data or an entire LIMS, maintaining an audit trail is an important part of [[21 CFR Part 11]] compliance.]] As codes and regulations like Title [[21 CFR Part 11]] mandate "computer systems (including hardware and software), controls, and attendant documentation" utilize electronic signatures and [[Audit trail|audit trails]]<ref>{{cite web |url=http://ecfr.gpoaccess.gov/cgi/t/text/text-idx?c=ecfr&sid=5ff3a0efed913ef8fae9e225869688a2&rgn=div5&view=text&node=21:1.0.1.1.7&idno=21 |title=Electronic Code of Federal Regulations - Title 21: Food and Drugs - Part 11: Electronic Records; Electronic Signatures |publisher=U.S. Government Printing Office |accessdate=02 March 2012}}</ref>, LIMS developers must put serious thought into how their software handles audit trail functionality. The audit trail — documentation of the sequence of activities that have affected an action — must be thorough and seamlessly integrated into the software. | ||
Information recorded in the audit trail typically includes: | Information recorded in the audit trail typically includes: | ||
| Line 270: | Line 316: | ||
===Chain of custody=== | ===Chain of custody=== | ||
The [[chain of custody]] (COC) of an item is of varying importance, depending on the type of laboratory. A highly regulated laboratory that works under Code of Federal Regulation or other guidelines makes tracking COC a vital part of its operations. This is especially true in forensic labs, which depend on continuous accountability of their evidence collection, retention, and disposal procedures.<ref>{{cite book |title=Building a Digital Forensic Laboratory: Establishing and Managing a Successful Facility |chapter=Chapter 1: An Introduction to Digital Forensics |author=Jones, Andrew; Craig Valli |url=http://books.google.com/books?id=F5IU7XXKwCQC |pages=11 |year=2008 |publisher=Butterworth-Heinemann |isbn=1856175103}}</ref> As with an [[audit trail]], a laboratory depends on recorded information like user ID, time stamp, and location ID to maintain a robust and accurate COC. [[ | The [[chain of custody]] (COC) of an item is of varying importance, depending on the type of laboratory. A highly regulated laboratory that works under Code of Federal Regulation or other guidelines makes tracking COC a vital part of its operations. This is especially true in forensic labs, which depend on continuous accountability of their evidence collection, retention, and disposal procedures.<ref>{{cite book |title=Building a Digital Forensic Laboratory: Establishing and Managing a Successful Facility |chapter=Chapter 1: An Introduction to Digital Forensics |author=Jones, Andrew; Craig Valli |url=http://books.google.com/books?id=F5IU7XXKwCQC |pages=11 |year=2008 |publisher=Butterworth-Heinemann |isbn=1856175103}}</ref> As with an [[audit trail]], a laboratory depends on recorded information like user ID, time stamp, and location ID to maintain a robust and accurate COC. [[LIMS feature#Barcode support|Barcodes]], [[LIMS feature#Inventory management|inventory management]], and [[LIMS feature#Configurable roles and security|configurable security roles]] all play an important part in maintaining chain of custody. | ||
===Configurable roles and security=== | ===Configurable roles and security=== | ||
Many roles exist within the laboratory setting, each with its own set of responsibilities. And just as the role an individual plays within the laboratory may change, so may the responsibilities associated with each role. This sort of change necessitates a flexible and configurable security system, one that allows for the placement of individual | Many roles exist within the laboratory setting, each with its own set of responsibilities. And just as the role an individual plays within the laboratory may change, so may the responsibilities associated with each role. This sort of change necessitates a flexible and configurable security system, one that allows for the placement of individual LIMS users into standardized security roles which provide role-specific access to certain LIMS functionality. Additionally, as responsibilities change within roles, that same flexible configuration is necessary for assigning or restricting access to certain LIMS functionality for each existing or newly created role. | ||
Of course, roles aren't always assigned on an individual level. Often large groups of individuals may need to be assigned to roles, necessitating group assignments for security purposes. For example, a group of laboratory trainees may only be given read-only access to the [[ | Of course, roles aren't always assigned on an individual level. Often large groups of individuals may need to be assigned to roles, necessitating group assignments for security purposes. For example, a group of laboratory trainees may only be given read-only access to the [[LIMS feature#Sample login and management|sample login]] and [[LIMS feature#Sample tracking|sample tracking]] functionality of the system through a custom "Trainees" group role, while the head researcher of the lab may be given the "Administrator" role, which allows that individual to access most if not all of the LIMS' functionality. | ||
===Data normalization=== | ===Data normalization=== | ||
For the purposes of describing | For the purposes of describing LIMS functionality, "data normalization" specifically refers to the process of ensuring incoming/imported data into the LIMS is standardized to the same format of existing LIMS data. | ||
Here's an example to better explain this issue. When | Here's an example to better explain this issue. When a LIMS is initially configured, in most if not all cases a clear standard can be set for how logged samples and their associated measurements pre- and post-analysis are recorded in the system. Perhaps all temperatures will be recorded in Celsius to two decimal places. If temperature data imported from a spreadsheet or a lab instrument is not in this format, the LIMS can normalize the incoming data to match the standard already set for existing LIMS temperature data. This ensures consistency within the LIMS database and typically leads to better data validation efforts later on. | ||
''Note'': Some | ''Note'': Some LIMS developers may include data normalization functionality within what they may refer to as data validation functionality. The line between these two may be blurred or not exist at all. | ||
===Data validation=== | ===Data validation=== | ||
For the purposes of describing | For the purposes of describing LIMS functionality, "data validation" specifically refers to the process of ensuring existing data in the LIMS — either pre-analysis or post-analysis — sufficiently meets any number of standards or thresholds set for sample login, sample analysis, or any other data management process in the LIMS. This validation process may be completely automatic and system-based, or it may also include additional steps on the part of the user base utilizing additional LIMS functionality, including verification of standard operating procedures (SOPs), QC samples, and QA approval.<ref>{{cite web |url=http://sirfer.utah.edu/qaqc.pdf |title=Quality Assurance - Data Management |publisher=Stable Isotope Ratio Facility for Environmental Research (SIRFER) at the University of Utah |accessdate=07 May 2012 |type=PDF}}</ref><ref>{{cite book |title=Application of Computers and Operations Research in the Mineral Industry |chapter=Chapter 10: Efficient utilization of LIMS data and integration with mining process management systems |author=Hitchcock, Noel |editor=Dessureault, Sean D.; Rajive Ganguli; Vladislav Kecojevic; Jami Girard Dwyer |url=http://www.crcnetbase.com/doi/abs/10.1201/9781439833407.ch10 |pages=85–88 |year=2005 |publisher=Taylor & Francis |isbn=9780415374491 |doi=10.1201/9781439833407.ch10}}</ref> | ||
''Note'': This functionality shouldn't be confused with the process of validating the | ''Note'': This functionality shouldn't be confused with the process of validating the LIMS itself, which is an entirely different process partially falling under [[LIMS feature#Regulatory compliance|regulatory compliance]] and involves the process of ensuring "the software is performing in a manner for which it was designed."<ref>{{cite journal |journal=Quality Assurance |year=2001 |volume=9 |issue=3–4 |pages=217–224 |title=Required steps for the validation of a Laboratory Information Management System |author=Turner, Elizabeth; Jojean Bolton |pmid=12553085 |url=http://www.ncbi.nlm.nih.gov/pubmed/12553085}}</ref> | ||
===Data encryption=== | ===Data encryption=== | ||
The existence of this functionality in LIMS software generally indicates the LIMS has the ability to protect the integrity and authenticity of its housed data through the use of a variety of technologies which makes data unreadable except to those possessing a key/right/etc. to unlock/read the data. This functionality is especially vital to the Web-enabled LIMS, which transfers information over the Internet in a client-server relationship. As a wide variety of encryption technologies exist, it's generally a good idea to consult with the developers of a LIMS to determine the strengths and weaknesses of their employed encryption methods. | |||
=== | ===Version control=== | ||
Version control is a form of safeguard which helps preserve data integrity. This is typically done by creating a modifiable new version of a piece of information rather than allowing the original to be modified. Such versioning may be applied to a wide variety of digital information housed in the LIMS, including test methods, training certifications, instrument logs, specifications, and process and procedure (P&P) documentation. In LIMS like [[LabWare,_Inc.#LabWare_LIMS|LabWare LIMS]], reference data can also be versioned while also retaining the original relationship between samples and test results, including the version of reference data current at the time lab testing is performed.<ref>{{cite web |url=http://www.labware.com/LWWeb.nsf/lp/en041001 |title=LabWare - Table Explorer |publisher=[[LabWare, Inc.]] |accessdate=07 May 2012}}</ref> Information tracked with such revisions includes attributes like user name, time the edit was made, and what exactly was edited. This also benefits those managing [[LIMS feature#Audit trail|audit trails]] and [[LIMS feature#Chain of custody|chains of custody]]. | |||
[[File:Freezers for cryopreservation at IMSC.JPG|thumb|The temperature of an open cryopreservation container may be monitored on a computer via a connection to a LIMS with environmental monitoring functionality.]] Other LIMS may employ a different form of version control called file locking, which simply puts the affected information into a read-only mode for users while someone else is busy editing it. Another popular strategy is to, rather than locking the file, allow multiple people edit to a piece of information, later merging the various edits. Potential LIMS buyers may need to inquire with developers to determine what type of versioning scheme is used in the vendor's software. | |||
=== | ===Automatic data backup=== | ||
The existence of this piece of functionality in a LIMS usually means information contained in one or more associated databases or [[LIMS feature#Data warehouse|data warehouses]] can be automatically preserved in an additional backup file. The save location for that file as well as the scheduled backup time is configurable, typically through the [[LIMS feature#Administrator management|administrative module]] of the LIMS. | |||
=== | ===Environmental monitoring=== | ||
Some LIMS like [[Core Informatics, LLC#Core LIMS|Core LIMS]] and [[Oracle Corporation#Oracle Health Sciences LabPas|Oracle Health Sciences LabPas]] allow users to monitor the environmental conditions of not only sample storage containers but also the entire laboratory itself.<ref>{{cite web |url=http://www.corelims.com/features.htm |title=Core LIMS Features & Components |publisher=[[Core Informatics, LLC]] |accessdate=07 May 2012}}</ref><ref>{{cite web |url=http://www.oracle.com/us/industries/life-sciences/health-sciences-labpas-ds-396097.pdf |title=Oracle Health Sciences LabPas |publisher=[[Oracle Corporation]] |date=2011 |accessdate=07 May 2012 |format=PDF}}</ref> Attributes like humidity, air quality, and temperature may be monitored to ensure sample storage units and experiments maintain desired conditions. Alarms may be able to be configured to notify staff if a storage container's environmental attributes go beyond a certain threshold. Manufacturers utilizing a LIMS like [[Novatek International#NOVA-LIMS|NOVA-LIMS]] may also be able to employ more advanced environmental tracking features in the plant to guarantee a more consistent, higher quality product is created.<ref>{{cite web |url=http://ntint.com/prod-envmon.shtml |title=Environmental Monitoring Program |publisher=[[Novatek International]] |accessdate=07 May 2012}}</ref> | |||
</div> | </div> | ||
| Line 322: | Line 366: | ||
===Custom reporting=== | ===Custom reporting=== | ||
Reporting | Reporting is a vital part of a LIMS, as it allows users to gain a clearer picture of collected data and potential trends. At a minimum a number of pre-configured report styles come standard with a LIMS. However, some LIMS are more flexible than others, offering the ability to customize reports in numerous ways. The most popular attributes of custom reporting include custom headers, custom information placement, charts, pivot tables, and multiple output formats. | ||
Note: Some | Note: Some LIMS vendors will offer custom reporting as an option as an added cost, depending on the level of customization required. | ||
===Report printing=== | ===Report printing=== | ||
Today's software almost universally offers the ability to print reports and other materials, so this feature may seem a bit redundant to list. Nonetheless, printer support is a feature worth confirming when considering a piece of | Today's LIMS software almost universally offers the ability to print reports and other materials, so this feature may seem a bit redundant to list. Nonetheless, printer support is a feature worth confirming when considering a piece of LIMS software. | ||
===Label support=== | ===Label support=== | ||
The label — typically affixed to a sample container | The label — typically affixed to a sample container — is a vital part of the sample tracking process.<ref name="SCLabel">{{cite web |url=http://www.scientificcomputing.com/bar-code-and-sample-tracking.aspx |title=Bar Code and Sample Tracking: It All Starts with the Label |author=Gilles, Clarence |publisher=Scientific Computing |date=1 July 2008 |accessdate=8 May 2012}}</ref> Identifying information such as sample number, batch number, and barcodes are printed on such labels to ensure optimized sample management and more precise sample data. As such, some LIMS allow users to design and print labels directly from the software. | ||
[[File:Code barres.png|thumb|left|The word "Wikipedia" encoded in Code 128 and Code 39]] | [[File:Code barres.png|thumb|left|The word "Wikipedia" encoded in Code 128 and Code 39]] | ||
===Barcode support=== | ===Barcode support=== | ||
Barcodes offer many advantages to laboratory techs handling samples, including more accurate data input, tighter sample/instrument associations, tighter sample/study associations, and more room for human-readable information on a label.<ref name="SCLabel" /> Given such advantages, many | Barcodes offer many advantages to laboratory techs handling samples, including more accurate data input, tighter sample/instrument associations, tighter sample/study associations, and more room for human-readable information on a label.<ref name="SCLabel" /> Given such advantages, many LIMS developers have integrated barcode support into their laboratory information management systems, including support for symbologies like Code 128, Code 39, and Interleaved 2 of 5. Aside from printing options, a LIMS may also offer support for a variety of bar code readers. | ||
Barcode support and [[ | Barcode support and [[LIMS feature#Label support|label support]] are typically found together in LIMS software, but not always, thus their separation into two features of a LIMS. | ||
===Export to PDF=== | ===Export to PDF=== | ||
A LIMS with this feature is able to collect and save information into a Portable Document Format (PDF). | |||
===Export to MS Word=== | ===Export to MS Word=== | ||
A LIMS with this feature is able to collect and save information into a Microsoft Office Word format. | |||
===Export to HTML or XML=== | ===Export to HTML or XML=== | ||
A LIMS with this feature is able to collect and save information into a HyperText Markup Language (HTML) and/or Extensible Markup Language (XML) format. | |||
===Fax integration=== | |||
A LIMS with this feature is able to connect with a fax machine and send information to it via manual input, automatically, and/or at scheduled intervals. | |||
===Email integration=== | ===Email integration=== | ||
A LIMS with this feature is able to integrate with and use the electronic mail information exchange method to send reports, alerts, and more via manual input, automatically, and/or at scheduled intervals. | |||
</div> | </div> | ||
| Line 367: | Line 415: | ||
===Administrator management=== | ===Administrator management=== | ||
The administrator management tools of | The administrator management tools of a LIMS allow lab technicians to set up the LIMS most optimally for the laboratory. Through the administrator management interface of a LIMS, other features may be accessed like setting up [[LIMS feature#Configurable roles and security|user roles]] and scheduling [[LIMS feature#Automatic data backup|automatic data backups]]. | ||
Like [[ | Like [[LIMS feature#Report printing|report printing]], administrator management is nearly ubiquitous in LIMS software, generally considered a mandatory feature. However, for the purposes of being thorough, it's important to point out its existence. | ||
===Modular=== | ===Modular=== | ||
This feature indicates that | This feature indicates that a LIMS has an intentional modular design, which separates some functionality into manageable components of the overall system. Generally speaking, a modular design allows for 1. the structured addition of new functionality to a LIMS and 2. the limiting of overall effects on the system design as new functionality is added. | ||
===Instrument interfacing and management=== | ===Instrument interfacing and management=== | ||
[[File: | [[File:Gas Chromatography Laboratory.jpg|thumb|260px|An entire room of [[gas chromatography]] instruments could potentially be connected to a LIMS via instrument interfacing.]]In laboratories there are instruments, and with those instruments comes scientific measurements which produce data. It's therefor natural a lab technician would want to connect those instruments to a laboratory information management system, which is already organizing and storing laboratory data. This sort of interfacing is typically handled with instrument-to-LIMS interfaces, which started out as merely data-transfer mechanisms. Later that interface mechanism became much more robust as a data management tool, though often at great expense with heavy involvement from third parties.<ref>{{cite web |url=http://www.scientific-computing.com/features/feature.php?feature_id=88 |title=Trends in instrument-to-LIMS interfacing |author=Pavlis, Robert |publisher=Scientific Computing World |date=May/June 2004 |accessdate=8 May 2012}}</ref> Today, "many LIMS vendors can act as single source providers of the entire instrument interfacing solution,"<ref>{{cite web |url=http://www.limsfinder.com/BlogDetail.aspx?id=33851_0_2_0_C |title=Instrument Interfacing - The Great Paradox of LIMS? |author=DeHeer, Larry |publisher=LIMSfinder.com |date=1 October 2009 |accessdate=8 May 2012}}</ref>, providing a cheaper and smoother solution to LIMS customers. | ||
===Mobile device integration=== | ===Mobile device integration=== | ||
While not | While not incredibly common, a few LIMS developers are including support for mobile devices in their laboratory information management system. [[AgileBio#LabCollector|LabCollector]], for example, extends its LIMS' functionality to Pocket PC or Windows CE devices equipped with wireless barcode scanners, allowing users to read or collect sample information while on the move.<ref>{{cite web |url=http://labcollector.com/index.php/labcollector-on-pdahtml |title=LabCollector - Features in detail |publisher=[[AgileBio]] |accessdate=8 May 2012}}</ref> Future Technologies' [[Future Technologies, Inc.#DNA LIMS|DNA LIMS]], designed for labs performing DNA analysis, has its own mobile version for technicians who need access but can't be in the lab.<ref>{{cite web |url=http://www.ftechi.com/dna_biometric.shtml |title=Biometrics and DNA Laboratory Information Management and Analysis System Development |publisher=[[Future Technologies, Inc.]] |accessdate=8 May 2012}}</ref> | ||
=== | ===Alarms and/or alerts=== | ||
Alarms and alerts are an integral part of a LIMS. They can be automatic or scheduled, and they can come in the form of an e-mail, a pop-up message, or a mobile text message. When the results for a sample analysis go out out of range, an automatic warning message can appear on the screen of the technician responsible for the analysis. A scheduled alert can be e-mailed to a lab technician every month indicating a piece of laboratory equipment needs routine maintenance. If the LIMS is equipped with [[LIMS feature#Environmental monitoring|environmental monitoring]], an alert can be sent in the form of an SMS text message to the head researcher if the temperature inside a freezer unexpectedly rises. All of these scenarios represent a tiny fraction of the possible implementation of alarms and alerts in a LIMS, highlighting how powerful (yet easy to take for granted) this feature is. | |||
===Work-related time tracking=== | |||
=== | This feature specifically refers to a LIMS' ability to track the amount of time an employee spends at work in general (for payroll purposes) or on more specific projects and tasks (as part of an employee work evaluation program). | ||
===Voice recognition system=== | |||
A LIMS with this feature allows some functions of the LIMS (for example, accessing sample analysis results) to be accessed via voice commands. | |||
===External monitoring=== | ===External monitoring=== | ||
This feature allows clients | This feature allows clients outside the laboratory to monitor the status of sample batches, test results, and more via an online Web portal or, less commonly, as activity alerts sent via e-mail, fax, or SMS. | ||
===Messaging=== | ===Messaging=== | ||
The messaging feature of | [[File:Example of Telecommunication Presence Information.png|thumb|Instant messaging clients built into a LIMS often make it easier to collaborate.]]The messaging feature of a LIMS may refer to one (or both) of two things: | ||
* a built-in instant messaging system that allows users to converse with each other through text messages real-time | * a built-in instant messaging system that allows users to converse with each other through text messages real-time | ||
* an SMS text messaging integration that allows the users or the | * an SMS text messaging integration that allows the users or the LIMS itself to send messages or alerts to a user's mobile or smart phone | ||
===Multilingual=== | ===Multilingual=== | ||
If | If a LIMS is listed as multilingual, its an indication the LIMS interface can be configured to display more than one language depending on the preference a user or administrator chooses. Some LIMS interfaces can only be displayed in one of two languages (English or German, for example), while others come configured with support for dozens of languages. | ||
===Network-capable=== | ===Network-capable=== | ||
This feature is perhaps archaic and/or obvious, but it is mentioned nonetheless. It's generally applied to a non- | This feature is perhaps archaic and/or obvious, but it is mentioned nonetheless. It's generally applied to a non-Web-based LIMS installed over a local or wide-area computer network, essentially indicating the LIMS is not an isolated application, but rather one that can interface with other instances of the LIMS or other networked instruments. | ||
===Web client or portal=== | ===Web client or portal=== | ||
A LIMS with a Web client or portal is either a Web-based LIMS (one that is not installed on every computer, but rather is hosted on a server and accessed via a Web browser) or a non-Web-based LIMS with an included portal to access it via the Internet. | |||
===Online or integrated help=== | ===Online or integrated help=== | ||
This indicates | This indicates a LIMS has help infrastructure integrated into the software, support documentation via the LIMS vendor's website, or both. | ||
===Software as a service delivery model=== | ===Software as a service delivery model=== | ||
| Line 437: | Line 476: | ||
===Usage-based cost=== | ===Usage-based cost=== | ||
While rare, some | While rare, some LIMS vendors allow potential clients to license and utilize the vendor's software under a usage-based cost model. An example of this model in use is Bytewize AB's [[Bytewize AB#O3 Lims and O3 LimsXpress|O3 LimsXpress]], which has a cost directly related to the amount of samples processed each month.<ref>{{cite web |url=http://www.bytewize.com/o3lims-xpress/prices/?lang=en |title=Modern web based Lims from 10 Euro cent/sample |publisher=[[Bytewize AB]] |accessdate=8 May 2012}}</ref> | ||
</div> | </div> | ||
Revision as of 16:49, 4 February 2016
|
|
You can find a listing of all ELN vendors — and by extension, the features their products offer — on the ELN vendor page. |
An ELN feature is one or more pieces of functionality that appear within an electronic laboratory notebook (ELN).
The ELN is an evolving concept, with new features and abilities being introduced every year. As laboratory demands change and technological progress continues, the functions of an ELN will also change. Yet like the dishwasher, the ELN tends to have a base set of functionality that defines it.
Electronic laboratory notebooks extend the functionality and efficiency of four central aspects of the researcher's activities[1]:
- recording experimental and other types of research data
- providing organized and searchable structure to that data
- allowing the sharing of that data through collaborations or with internal or external individuals
- communicating with others about that data and research
Of course, there are ELN features that are difficult to categorize under any of these activities. Such features often contribute to the entire ELN and how it's utilized. For example, multilingual support allows users to interact with the ELN in more than one language. Some functionality may also overlap several research phases, making it difficult to firmly classify.
The features described below come from an analysis of freely available ELN product information on vendor websites. An attempt was made to discover the features most utilized in vendors' ELN products and collect information on those features for each ELN. Not every possible feature is referenced here; some ELN products fill specific niches, utilizing unique functionality to solve a specific problem.
That said, keep in mind the categorization of features below is very loose. It may be viable to argue a feature belongs under a different section or multiple sections. For the purposes of organizing this information in an uncomplicated manner, however, some liberty has been taken in the categorizing of features.
Experiment, collaboration, and data management
To hide the contents of this section for easier reading of other sections, click the "Collapse" link to the right.
Chemical drawing and calculation
Chemical and/or spectrum file support
Reagents database
Task and event scheduling
Within the context of an ELN, the ability to schedule a task or event is a natural extension of how work was done in a laboratory before the advent of data management systems. Experiments are assigned to technicians, maintenance schedules are created and followed, and research deadlines must be observed. While these tasks have in the past been performed without ELN, a modern data management system can now optimize those tasks and provide additional scheduling functionality to streamline the operation of a lab. Some ELNs like Labtronics Inc.'s Nexxis ELN can schedule due dates on worksheets and experiments.[2] Additional functionality within this feature group includes the ability to configure automated assignments of experiment requests, establish recurring events, and in most cases, create printable reports.
Examples of tasks and events that can feasibly be scheduled in an ELN include:
- production of reports
- creation and sending of e-mails and alerts
- maintenance of equipment
- assignment of experiments to personnel
Option for manual result entry
While many ELN vendors tout the ability of their product to automate the entry of experiment results into the ELN database, the need for manual data entry of analysis results still exists. This feature is important to laboratories obtaining analysis results from multiple sources, including non-digital paper-based results and instruments that can't be connected to the ELN. Additional functionality associated with this feature includes a customizable spell check dictionary and the ability to add comments, notes, and narratives to most anything in the ELN.
Multiple data viewing methods
Researchers produce reams of data, and the ELN exists to help organize and share that data. Additionally, even before the existence of the ELN, scientists have had a corresponding need for visually representing data. Today an ELN can not only collect and analyze data from experiments, but it also can represent that data in reports, graphs, gradients, and spreadsheets. Depending on the ELN, more than one way to visually represent the data may exist.
The ELN is particularly useful for displaying chemical structures and bonds in more than one way through built-in or integratable chemical structure drawing tools or through a document manager that can handle the placement of screenshots, sketches, or other images.
Data and trend analysis
Sample experimentation and analysis plays an important part of laboratory informatics, helping laboratories make better sense of their experiments and reach valuable conclusions about them. While this important phase of laboratory work has often been done externally from the ELN, it's now more common to see basic analysis tools being included. Such tools allow raw data to be imported directly to the ELN, which then can store, process, and display it in a shareable form. Additionally, chemical calculations and functions used in the analysis are typically definable and editable for further flexibility.As with the feature "multiple data viewing methods," data and trend analysis is also increasingly important in laboratories that have very specialized data management needs. When even in 2009 genetic scientists in large- and medium-sized sequencing and core centers were voicing concerns about "a lack of adequate vendor-supported software and laboratory information management systems (LIMS),"[3], today data management options like Scilligence ELN are starting to emerge, offering the ability to perform specialized analytical tasks for the researcher.[4]
As sample experimentation and data analysis are important parts of most if not all laboratories, such functionality — which has often come in the form of a separate application or analysis device — will likely continue to merge into software like ELN, LIMS, and other laboratory informatics solutions.[5]
Data and equipment sharing
Aside from data storage and sample registration, a modern ELN's major contribution to the laboratory is both in aiding in the sharing of experiment results, reports, and other research data with those who need it most and the documentation of vital evidence for potential patent interference cases. Rather than pieces of information becoming misplaced or forgotten in physical laboratory notebooks, the ELN makes it easier to share experiment results and increase the efficiency of collaboration inside and outside the laboratory. Yet data is more than just test results; it also can come in the form of charts, reports, policy and procedure, and other documents. Additionally, the need for controlling who has access to those types of data is also an important consideration. As such, this feature is at least partially tied to other features like document management and configurable security.
Customizable fields and/or interface
As thorough as some user interface (UI) developers may be in adding relevant fields and interface options for eln end users, there are at times options that are either omitted or unanticipated. This has traditionally required the end user to contact the vendor and ask if the needed option(s) can be added in the next release. However, many modern ELN vendors have responded instead by adding functionality that gives end users and/or ELN administrators more control over the user interface.
Aspects of the ELN's user interface that are often customizable by the end user include:Cite error: Closing </ref> missing for <ref> tag[6] Finally, as LIMS continue to include both sample management and experimental data management functionality, queries become more powerful in general as now sample and experiment can be matched together in one database.[5]
Query functionality often includes the ability to:
- search both transactional data and archived data tables
- search multiple databases via an application programming interface (API) or open database connectivity (ODBC) connection
- filter and sort data
- create ad-hoc queries
Import data
Data can originate from numerous places in the laboratory. The ability to import that data into a LIMS can be beneficial, especially when an instrument can't be connected or an external client provides a data feed independent of the LIMS. Some LIMS like Bridge-Soft's QMS even allow to cross-reference laboratory nomenclature from received data sources with the recipient's.[7] And of course instrument interfacing allows for even more importation options. Additional data validation procedures may be applied to the imported data to guarantee information homogeneity. Additionally, some LIMS may allow for the import and integration of non-normalized legacy data tables with LIMS data tables into a single database.
Internal file or data linking
This feature allows LIMS users to link together reports, protocols, sample results, and more, providing greater contextual clarity to projects. Examples include:
- linking a sample batch to a test or sample preparation methodology
- linking a test process to a particular customer
- linking a report to a sample batch
- linking a group of test results to a raw data file
- linking an image to a work order
- linking all lab results with the correct reporting test method
External file or data linking
This feature allows LIMS users to link together data and files in the LIMS with data, files, and customers outside the scope of the LIMS. Examples include:
- linking data files from chromatography equipment to synthesis data[8]
- linking equipment ID with an external annotation database[9]
- linking external standard operating procedure documents with an internal test specification[10]
ELN support or integration
As a software replacement for more traditional paper laboratory notebooks, the electronic laboratory notebook (ELN) has been important to laboratory functions. Yet the lines between ELN and LIMS began to blur in the 2000s, with both types of software incorporating features from the other.[11][12] The result today is some LIMS either include traditional ELN functionality or link physical laboratory notebook references to data in the LIMS.[13]
Export to MS Excel
While Microsoft Excel has long been used within the laboratory setting, a slow shift towards relational databases and LIMS occurred in the late 1990s and early 2000s.[14] Additional concerns with the difficulties of Excel's validation and compliance with FDA 21 CFR Part 11 and other regulations have led many labs to turn to data management solutions that are easier to validate.[15] Nevertheless, laboratories continue to use Excel in some fashion, and thus Excel integration or data exportation in Excel format is a real need for LIMS customers. LIMS with this feature allow raw, processed, or imported data to be exported in the Excel format for further analysis and dissemination.
Raw data management
While not described as a feature on most LIMS vendor websites, a few indicate that their LIMS is capable of managing (import, export, editing, etc.) data in its raw format for future analysis.
Data warehouse
A LIMS' data warehouse serves the important function of storing, extracting, and managing the data that laboratories crank out for the purposes or analysis, reporting, and process management, typically separate from the primary storage database. Data warehouses also offer the benefit of speeding up queries, making queries and data mining more user-friendly, and smoothing out data gaps.[16]
Deadline control
Deadline control is functionality within a LIMS that allows users to manage and be notified of events that occur within the laboratory. With this functionality users can also be notified of upcoming deadlines on anything from sample analysis to license renewal.
Note that this functionality may also feasibly fall under the task and event scheduling or alarms features. As deadline control seems to be advertised as a notable feature by only a few vendors, it seems even more likely that this functionality is considered part of scheduling or alarms.
Production control
There are many types of businesses that produce goods, and in most cases there is a research laboratory involved at some point in the process. This is especially true in the pharmaceutical and chemical industries, where production measurements such as yield, volume, activity, and impurity are vital. As LIMSs have already recorded such information during tests and analysis, the addition of production control functionality seems natural. Some LIMS take a very active approach to this. For example, 2nd Sight Solutions' OhNo! features production control as major functionality for the synthesis of radiopharmaceuticals.[17] Other LIMS may have less pronounced production functionality, while still offering the ability to track the production process in and out of the lab. And yet other LIMSs like dialog's diaLIMS offer robust production-based functionality but as a module or add-on to the base LIMS software.[18]
The types of functionality that may fall under this feature include:
- recipe management
- consumable tracking
- batch traceability
- production planning
- enterprise resource planning
Project and/or task management
Project and task management within a LIMS typically involves the scheduling of tasks to workers and organizing associated tasks into a more cohesive unit for better tracking and management. While the functionality of task and event scheduling can also be found in project and task management, many LIMS include functionality beyond scheduling that warrants the addition of the project and/or task management feature. This functionality includes:
- job allocation and rescheduling
- instrument workload tracking
- time tracking
- pending workload verification
- priority setting
- project-based workflow management
- sample, batch, and document linking
- work list sharing
- recurring event management
Inventory management
- register origin, demographics of incoming materials
- track used and in-use items via barcodes
- track inventory reduction based on usage and shipping out of the lab
- create alerts for when items reach a certain stock level
- calculate inventory cost and fluctuation
- manage transportation and routing
- manual incrementing/decrementing of items
- track location and usage of laboratory equipment
- assign storage locations
- track forensic evidence
It should be noted that samples and electronic equipment may also be considered inventory, and thus there is likely some functionality crossover with the sample management and instrument management features.
Document creation and management
Standard operation procedures, (SOPs), specifications, reports, graphs, images, and receipts are all collected and used in the average laboratory. With a LIMS already designed to manage and store sample and experiment data, it makes sense to include functionality to create, import, export, and manage other sorts of data files. As sample and experimental data can be indexed, queried, and linked, so too can document data. Functionality of a typical document management system includes the ability to:
- upload and index documents
- enforce version control
- provide full text search
- export to PDF or other relevant format
- add documents as attachments
Case management
The laboratory information system (LIS) has played an important role in the case management tasks of patient-centric and clinical laboratories. However, some LIMS have gained case management functionality, effectively blurring the lines between LIS and LIMS.[19]. Self-proclaimed LIMS products have emerged in the clinical, public health, and veterinary industries, areas that have historically been served by LIS software. When also considering the fields of law enforcement and forensic science, case management has an increasingly important role in some LIMS. Functionality seen in the case management feature includes:
- case accessioning and assignment
- disease tracking
- trend analysis
- clinical history follow-up
- out-of-range result alerts
- document and result association
- evidence control
- study management
Workflow management
Workflow management is common in the laboratory, acting as a graphical representation of planned sequential steps to either fully or partially automate a process within the lab. Separate standards-based workflow management systems (in the form of a software component) have traditionally performed this task.[20] However, in the 2000s LIMS vendors began incorporating workflow management functionality into their LIMS software, reducing the headaches that customization of a LIMS often brought.[21]
Modern commercial and open-source LIMS solutions often feature workflow management functionality, including[22][23][21]:
- attribute definition of activities
- definition of inputs and outputs of activities
- assignment of documentation to activities
- setting of quality control limits
- dynamically modify workflow in case of future changes
- receive notification of changes to the workflow
- sending user-defined messages during the process
Specification management
Specification (spec) management is vital to not only the manufacturing and research industries but also to a host of other laboratories requiring precise measurements and infallible test methods. Just as the ASTM offers standards and specs for LIMS[24], so too do LIMS users have standards and specs for their laboratory. With spec management in place within the LIMS, laboratories can then:
- enforce standard operating procedures and business rules
- create specs down to a project or sample level
- validate recipes and procedures
- accept or reject sample batches
- document internal and external spec history
Note that some of the functionality of spec management may cross over into the realm of quality control and data validation.
Customer and supplier management
Unless a laboratory is conducting internalized independent research, in most cases it will do business with external entities such as contract labs, sample providers, equipment providers, and reagent suppliers. In some cases, even internal employees may be considered a customer, necessitating documentation of who is using the system and in what ways. For a veterinary lab, the customer may be the animal and handler. For a forensic lab the customer may be more complex: internal staff, university staff, police departments, and maintainers of nationwide crime databases may all at some point act as customers. In these cases, documenting these various points of contact and linking them to samples, equipment, and tests becomes vital. Managing demographics, complaints, correspondence, and history are all feasible with customer management functionality. This process is often made simpler through the use of a more context-neutral entity creation system, which allows for more flexible management of contacts.
This feature may also be referred to as contact management, an address book module, or a customer service module.
Billing management
While the finances of a laboratory are important, they've typically been handled separately as a business process. However, some LIMS include additional functionality to make handling financial transactions and documentation of all sorts possible within the LIMS. In theory, such functionality brings the possibility of keeping more of a laboratory's data centrally located and queryable. This feature may include:
- payment processing
- expense reporting
- price quotes
- revenue management
- workload tracking of billable hours
- bill of materials
- grant management
Quality, security, and compliance
To hide the contents of this section for easier reading of other sections, click the "Collapse" link to the right.
Regulatory compliance
The topic of whether or not a LIMS meets regulatory compliance is often a complex one. While Title 21 CFR Part 11 has arguably had the largest influence on a electronic data management system's compliance, other influential standards have shaped the way LIMS and other systems handle and store data. Other compliance-based codes, standards, and regulations include:
- ASTM
- ASCLD/LAB
- Classified data
- Freedom of information legislation (various)
- GALP and GAMP
- HIPAA
- Health Level 7
- ISO/IEC 17025
- ISO 9000/9001
- ISO/TS 16949
- ODBC
- TNI and NELAP
- Title 40 CFR Part 3
With so many codes, standards, and regulations, LIMS consumers are advised to contact vendors with their user requirements and ask how the vendor's software meets and/or exceeds those requirements.
QA/QC functions
The quality management functions of a LIMS allow users to maintain a level of necessary quality across many of the functions in a laboratory. From running quality assurance tests to ensuring employed researchers are proficient at certain tasks, the QA/QC functionality of a LIMS is largely responsible for the output of consistent data and manufactured products in and out of the lab.
Common functionality includes[25][26]:
- single or batch QA/QC tests
- quality control charts and reports
- proficiency testing
- document management
- instrument maintenance
- data acceptance/rejection
- certificates of analysis (COA)
- data types defined by QC analysis
Performance evaluation
As document management becomes increasingly prevalent in LIMS, it only makes sense to also collate and store all the documentation associated with employee training and certification. Changes to laboratory techniques, scientific understanding, and business practices force researchers to learn, reevaluate, and demonstrate competency in order to maintain quality levels in the laboratory. Evaluations can frequently extend beyond staff members, however. Clinics, visit types, vendors, or test species can also be tracked and evaluated based on custom criteria. The performance evaluation functionality of a LIMS makes this possible.
That functionality typically includes the ability to maintain training records and history, and also to link that training to a technique or piece of equipment. Afterwards, the staff member, vendor, etc. can be marked as competent or certified in the equipment, knowledge, or process. Periodical assessment of the training and its practical effectiveness can later be performed. Productivity of an entity or process can also be gauged over a certain date range based on tracked time, pre-determined milestones, or some other criteria.
Audit trail

Information recorded in the audit trail typically includes:
- operator code
- time stamp
- location
- case number
- transaction type
- amount and quantity prior to change
- user notes
Chain of custody
The chain of custody (COC) of an item is of varying importance, depending on the type of laboratory. A highly regulated laboratory that works under Code of Federal Regulation or other guidelines makes tracking COC a vital part of its operations. This is especially true in forensic labs, which depend on continuous accountability of their evidence collection, retention, and disposal procedures.[28] As with an audit trail, a laboratory depends on recorded information like user ID, time stamp, and location ID to maintain a robust and accurate COC. Barcodes, inventory management, and configurable security roles all play an important part in maintaining chain of custody.
Configurable roles and security
Many roles exist within the laboratory setting, each with its own set of responsibilities. And just as the role an individual plays within the laboratory may change, so may the responsibilities associated with each role. This sort of change necessitates a flexible and configurable security system, one that allows for the placement of individual LIMS users into standardized security roles which provide role-specific access to certain LIMS functionality. Additionally, as responsibilities change within roles, that same flexible configuration is necessary for assigning or restricting access to certain LIMS functionality for each existing or newly created role.
Of course, roles aren't always assigned on an individual level. Often large groups of individuals may need to be assigned to roles, necessitating group assignments for security purposes. For example, a group of laboratory trainees may only be given read-only access to the sample login and sample tracking functionality of the system through a custom "Trainees" group role, while the head researcher of the lab may be given the "Administrator" role, which allows that individual to access most if not all of the LIMS' functionality.
Data normalization
For the purposes of describing LIMS functionality, "data normalization" specifically refers to the process of ensuring incoming/imported data into the LIMS is standardized to the same format of existing LIMS data.
Here's an example to better explain this issue. When a LIMS is initially configured, in most if not all cases a clear standard can be set for how logged samples and their associated measurements pre- and post-analysis are recorded in the system. Perhaps all temperatures will be recorded in Celsius to two decimal places. If temperature data imported from a spreadsheet or a lab instrument is not in this format, the LIMS can normalize the incoming data to match the standard already set for existing LIMS temperature data. This ensures consistency within the LIMS database and typically leads to better data validation efforts later on.
Note: Some LIMS developers may include data normalization functionality within what they may refer to as data validation functionality. The line between these two may be blurred or not exist at all.
Data validation
For the purposes of describing LIMS functionality, "data validation" specifically refers to the process of ensuring existing data in the LIMS — either pre-analysis or post-analysis — sufficiently meets any number of standards or thresholds set for sample login, sample analysis, or any other data management process in the LIMS. This validation process may be completely automatic and system-based, or it may also include additional steps on the part of the user base utilizing additional LIMS functionality, including verification of standard operating procedures (SOPs), QC samples, and QA approval.[29][30]
Note: This functionality shouldn't be confused with the process of validating the LIMS itself, which is an entirely different process partially falling under regulatory compliance and involves the process of ensuring "the software is performing in a manner for which it was designed."[31]
Data encryption
The existence of this functionality in LIMS software generally indicates the LIMS has the ability to protect the integrity and authenticity of its housed data through the use of a variety of technologies which makes data unreadable except to those possessing a key/right/etc. to unlock/read the data. This functionality is especially vital to the Web-enabled LIMS, which transfers information over the Internet in a client-server relationship. As a wide variety of encryption technologies exist, it's generally a good idea to consult with the developers of a LIMS to determine the strengths and weaknesses of their employed encryption methods.
Version control
Version control is a form of safeguard which helps preserve data integrity. This is typically done by creating a modifiable new version of a piece of information rather than allowing the original to be modified. Such versioning may be applied to a wide variety of digital information housed in the LIMS, including test methods, training certifications, instrument logs, specifications, and process and procedure (P&P) documentation. In LIMS like LabWare LIMS, reference data can also be versioned while also retaining the original relationship between samples and test results, including the version of reference data current at the time lab testing is performed.[32] Information tracked with such revisions includes attributes like user name, time the edit was made, and what exactly was edited. This also benefits those managing audit trails and chains of custody.
Automatic data backup
The existence of this piece of functionality in a LIMS usually means information contained in one or more associated databases or data warehouses can be automatically preserved in an additional backup file. The save location for that file as well as the scheduled backup time is configurable, typically through the administrative module of the LIMS.
Environmental monitoring
Some LIMS like Core LIMS and Oracle Health Sciences LabPas allow users to monitor the environmental conditions of not only sample storage containers but also the entire laboratory itself.[33][34] Attributes like humidity, air quality, and temperature may be monitored to ensure sample storage units and experiments maintain desired conditions. Alarms may be able to be configured to notify staff if a storage container's environmental attributes go beyond a certain threshold. Manufacturers utilizing a LIMS like NOVA-LIMS may also be able to employ more advanced environmental tracking features in the plant to guarantee a more consistent, higher quality product is created.[35]
Reporting, barcoding, and printing
To hide the contents of this section for easier reading of other sections, click the "Collapse" link to the right.
Custom reporting
Reporting is a vital part of a LIMS, as it allows users to gain a clearer picture of collected data and potential trends. At a minimum a number of pre-configured report styles come standard with a LIMS. However, some LIMS are more flexible than others, offering the ability to customize reports in numerous ways. The most popular attributes of custom reporting include custom headers, custom information placement, charts, pivot tables, and multiple output formats.
Note: Some LIMS vendors will offer custom reporting as an option as an added cost, depending on the level of customization required.
Report printing
Today's LIMS software almost universally offers the ability to print reports and other materials, so this feature may seem a bit redundant to list. Nonetheless, printer support is a feature worth confirming when considering a piece of LIMS software.
Label support
The label — typically affixed to a sample container — is a vital part of the sample tracking process.[36] Identifying information such as sample number, batch number, and barcodes are printed on such labels to ensure optimized sample management and more precise sample data. As such, some LIMS allow users to design and print labels directly from the software.
Barcode support
Barcodes offer many advantages to laboratory techs handling samples, including more accurate data input, tighter sample/instrument associations, tighter sample/study associations, and more room for human-readable information on a label.[36] Given such advantages, many LIMS developers have integrated barcode support into their laboratory information management systems, including support for symbologies like Code 128, Code 39, and Interleaved 2 of 5. Aside from printing options, a LIMS may also offer support for a variety of bar code readers.
Barcode support and label support are typically found together in LIMS software, but not always, thus their separation into two features of a LIMS.
Export to PDF
A LIMS with this feature is able to collect and save information into a Portable Document Format (PDF).
Export to MS Word
A LIMS with this feature is able to collect and save information into a Microsoft Office Word format.
Export to HTML or XML
A LIMS with this feature is able to collect and save information into a HyperText Markup Language (HTML) and/or Extensible Markup Language (XML) format.
Fax integration
A LIMS with this feature is able to connect with a fax machine and send information to it via manual input, automatically, and/or at scheduled intervals.
Email integration
A LIMS with this feature is able to integrate with and use the electronic mail information exchange method to send reports, alerts, and more via manual input, automatically, and/or at scheduled intervals.
Base functionality
To hide the contents of this section for easier reading of other sections, click the "Collapse" link to the right.
Administrator management
The administrator management tools of a LIMS allow lab technicians to set up the LIMS most optimally for the laboratory. Through the administrator management interface of a LIMS, other features may be accessed like setting up user roles and scheduling automatic data backups.
Like report printing, administrator management is nearly ubiquitous in LIMS software, generally considered a mandatory feature. However, for the purposes of being thorough, it's important to point out its existence.
Modular
This feature indicates that a LIMS has an intentional modular design, which separates some functionality into manageable components of the overall system. Generally speaking, a modular design allows for 1. the structured addition of new functionality to a LIMS and 2. the limiting of overall effects on the system design as new functionality is added.
Instrument interfacing and management

Mobile device integration
While not incredibly common, a few LIMS developers are including support for mobile devices in their laboratory information management system. LabCollector, for example, extends its LIMS' functionality to Pocket PC or Windows CE devices equipped with wireless barcode scanners, allowing users to read or collect sample information while on the move.[39] Future Technologies' DNA LIMS, designed for labs performing DNA analysis, has its own mobile version for technicians who need access but can't be in the lab.[40]
Alarms and/or alerts
Alarms and alerts are an integral part of a LIMS. They can be automatic or scheduled, and they can come in the form of an e-mail, a pop-up message, or a mobile text message. When the results for a sample analysis go out out of range, an automatic warning message can appear on the screen of the technician responsible for the analysis. A scheduled alert can be e-mailed to a lab technician every month indicating a piece of laboratory equipment needs routine maintenance. If the LIMS is equipped with environmental monitoring, an alert can be sent in the form of an SMS text message to the head researcher if the temperature inside a freezer unexpectedly rises. All of these scenarios represent a tiny fraction of the possible implementation of alarms and alerts in a LIMS, highlighting how powerful (yet easy to take for granted) this feature is.
This feature specifically refers to a LIMS' ability to track the amount of time an employee spends at work in general (for payroll purposes) or on more specific projects and tasks (as part of an employee work evaluation program).
Voice recognition system
A LIMS with this feature allows some functions of the LIMS (for example, accessing sample analysis results) to be accessed via voice commands.
External monitoring
This feature allows clients outside the laboratory to monitor the status of sample batches, test results, and more via an online Web portal or, less commonly, as activity alerts sent via e-mail, fax, or SMS.
Messaging
The messaging feature of a LIMS may refer to one (or both) of two things:- a built-in instant messaging system that allows users to converse with each other through text messages real-time
- an SMS text messaging integration that allows the users or the LIMS itself to send messages or alerts to a user's mobile or smart phone
Multilingual
If a LIMS is listed as multilingual, its an indication the LIMS interface can be configured to display more than one language depending on the preference a user or administrator chooses. Some LIMS interfaces can only be displayed in one of two languages (English or German, for example), while others come configured with support for dozens of languages.
Network-capable
This feature is perhaps archaic and/or obvious, but it is mentioned nonetheless. It's generally applied to a non-Web-based LIMS installed over a local or wide-area computer network, essentially indicating the LIMS is not an isolated application, but rather one that can interface with other instances of the LIMS or other networked instruments.
Web client or portal
A LIMS with a Web client or portal is either a Web-based LIMS (one that is not installed on every computer, but rather is hosted on a server and accessed via a Web browser) or a non-Web-based LIMS with an included portal to access it via the Internet.
Online or integrated help
This indicates a LIMS has help infrastructure integrated into the software, support documentation via the LIMS vendor's website, or both.
Software as a service delivery model
This indicates the software can be licensed and utilized via the software as a service (SaaS) delivery model.
Usage-based cost
While rare, some LIMS vendors allow potential clients to license and utilize the vendor's software under a usage-based cost model. An example of this model in use is Bytewize AB's O3 LimsXpress, which has a cost directly related to the amount of samples processed each month.[41]
References
- ↑ Macneil, Rory (6 October 2010). "What are electronic lab notebooks for?". Axiope. http://elnblog.axiope.com/?p=451. Retrieved 04 May 2011.
- ↑ "Nexxis Electronic Laboratory Notebook" (PDF). PerkinElmer, Inc. 2011. http://www.cambridgesoft.com/literature/PDF/Nexxis%20ELN.pdf. Retrieved 07 February 2013.
- ↑ Richter, Brent G.; David P. Sexton (2009). "Managing and Analyzing Next-Generation Sequence Data". PLoS Computational Biology 5 (6). doi:10.1371/journal.pcbi.1000369. http://www.ploscompbiol.org/article/info%3Adoi%2F10.1371%2Fjournal.pcbi.1000369.
- ↑ "Scilligence ELN". Scilligence Corporation. http://www.scilligence.com/web/eln.aspx. Retrieved 07 February 2013.
- ↑ 5.0 5.1 Macneil, Rory (2011). "The benefits of integrated systems for managing both samples and experimental data: An opportunity for labs in universities and government research institutions to lead the way". Automated Experimentation 3 (2). doi:10.1186/1759-4499-3-2. http://www.aejournal.net/content/3/1/2.
- ↑
- ↑
- ↑
- ↑
- ↑
- ↑ Elliot, Michael H. (December 2006–January 2007). "The state of the ELN Market". Scientific Computing World. http://www.scientific-computing.com/features/feature.php?feature_id=50. Retrieved 04 May 2011.
- ↑ Elliot, Michael H. (October 2011). "Informatics Convergence Presents Opportunities and Challenges". Scientific Computing. http://www.scientificcomputing.com/articles-IN-Informatics-Convergence-Presents-Opportunities-and-Challenges-111111.aspx. Retrieved 25 February 2012.
- ↑
- ↑ Williams, Robert W. (2003). "Managing Your Lab Data Flux: Getting Beyond Excel" (PDF). The Bioinformatics of Brains: From Genes and Proteins to Behaviors. Washington, DC: Society for Neuroscience. http://www.sfn.org/skins/main/pdf/ShortCourses/2003/sc1_9.pdf. Retrieved 17 February 2012.
- ↑ Howard, David A.; David Harrison (2007). "A Pragmatic Approach to the Validation of Excel Spreadsheets – Overview" (PDF). Pharma IT 1 (2): 30–35. http://www.spreadsheetvalidation.com/pdf/Excel_Spreadsheet_Validation_Overview.pdf.
- ↑ Vannest, Jeff (11 February 2011). "Advantages of a Data Warehouse". LABVANTAGE Solutions, Inc.. http://www.labvantage.com/blog/?p=79. Retrieved 17 February 2012.
- ↑
- ↑
- ↑ Hice, Randy (1 July 2009). "Swimming in the Clinical Pool: Why LIMS are supplanting old-school clinical LIS applications". STARLIMS' Laboratory Informatics Blog. http://blog.starlims.com/2009/07/01/swimming-in-the-clinical-pool-why-lims-are-supplanting-old-school-clinical-lis-applications/. Retrieved 09 May 2011.
- ↑ "Workflow Management Coalition Terminology & Glossary" (PDF). Workflow Management Coalition. February 1999. pp. 9. http://www.wfmc.org/standards/docs/TC-1011_term_glossary_v3.pdf. Retrieved 20 February 2012.
- ↑ 21.0 21.1 Maxwell, Glen (1 November 2003). "Using Workflows in LIMS to Reduce Customization". Scientific Computing and Instrumentation. http://www.scientificcomputing.com/using-workflows-in-lims-to-reduce.aspx. Retrieved 20 February 2012.
- ↑ Melo, Alexandre; Alessandra Faria-Campos; Daiane M DeLaat; Rodrigo Keller; Vinícius Abreu; Sérgio Campos (2010). "SIGLa: an adaptable LIMS for multiple laboratories". BMC Genomics 11 (Suppl 5): S8. doi:10.1186/1471-2164-11-S5-S8. http://www.biomedcentral.com/1471-2164/11/S5/S8.
- ↑ "My laboratory has unique processes, Can X-LIMS work for me?". EthoSoft, Inc.. http://www.ethosoft.com/LIMS/XLIMSProductInfo/XLIMSFAQ/tabid/69/Default.aspx. Retrieved 20 February 2012.
- ↑ "ASTM E1578 - 06 Standard Guide for Laboratory Information Management Systems (LIMS)". ASTM International. http://www.astm.org/Standards/E1578.htm. Retrieved 22 February 2012.
- ↑ Paszko, Christine; Carol Pugsley (2000). "Considerations in selecting a laboratory information management system (LIMS)". American Laboratory 32 (18): 38–42. doi:10.1016/0169-7439(94)90006-X. http://www.mendeley.com/research/considerations-selecting-laboratory-information-management-system-lims/.
- ↑ Hull, Carl; Bruce Wray; Ford Winslow; Mark Vilicich (2011). "Tracking and Controlling Everything that Affects Quality is the Key to a Quality Management System" (PDF). Combinatorial Chemistry & High Throughput Screening 14 (9): 772–780. doi:10.2174/138620711796957125. http://www.uniconnect.com/wp-content/uploads/2011/11/CCHTS-LIMS-Article.pdf.
- ↑ "Electronic Code of Federal Regulations - Title 21: Food and Drugs - Part 11: Electronic Records; Electronic Signatures". U.S. Government Printing Office. http://ecfr.gpoaccess.gov/cgi/t/text/text-idx?c=ecfr&sid=5ff3a0efed913ef8fae9e225869688a2&rgn=div5&view=text&node=21:1.0.1.1.7&idno=21. Retrieved 02 March 2012.
- ↑ Jones, Andrew; Craig Valli (2008). "Chapter 1: An Introduction to Digital Forensics". Building a Digital Forensic Laboratory: Establishing and Managing a Successful Facility. Butterworth-Heinemann. pp. 11. ISBN 1856175103. http://books.google.com/books?id=F5IU7XXKwCQC.
- ↑ "Quality Assurance - Data Management". Stable Isotope Ratio Facility for Environmental Research (SIRFER) at the University of Utah. http://sirfer.utah.edu/qaqc.pdf. Retrieved 07 May 2012.
- ↑ Hitchcock, Noel (2005). "Chapter 10: Efficient utilization of LIMS data and integration with mining process management systems". In Dessureault, Sean D.; Rajive Ganguli; Vladislav Kecojevic; Jami Girard Dwyer. Application of Computers and Operations Research in the Mineral Industry. Taylor & Francis. pp. 85–88. doi:10.1201/9781439833407.ch10. ISBN 9780415374491. http://www.crcnetbase.com/doi/abs/10.1201/9781439833407.ch10.
- ↑ Turner, Elizabeth; Jojean Bolton (2001). "Required steps for the validation of a Laboratory Information Management System". Quality Assurance 9 (3–4): 217–224. PMID 12553085. http://www.ncbi.nlm.nih.gov/pubmed/12553085.
- ↑ "LabWare - Table Explorer". LabWare, Inc.. http://www.labware.com/LWWeb.nsf/lp/en041001. Retrieved 07 May 2012.
- ↑ "Core LIMS Features & Components". Core Informatics, LLC. http://www.corelims.com/features.htm. Retrieved 07 May 2012.
- ↑ "Oracle Health Sciences LabPas" (PDF). Oracle Corporation. 2011. http://www.oracle.com/us/industries/life-sciences/health-sciences-labpas-ds-396097.pdf. Retrieved 07 May 2012.
- ↑ "Environmental Monitoring Program". Novatek International. http://ntint.com/prod-envmon.shtml. Retrieved 07 May 2012.
- ↑ 36.0 36.1 Gilles, Clarence (1 July 2008). "Bar Code and Sample Tracking: It All Starts with the Label". Scientific Computing. http://www.scientificcomputing.com/bar-code-and-sample-tracking.aspx. Retrieved 8 May 2012.
- ↑ Pavlis, Robert (May/June 2004). "Trends in instrument-to-LIMS interfacing". Scientific Computing World. http://www.scientific-computing.com/features/feature.php?feature_id=88. Retrieved 8 May 2012.
- ↑ DeHeer, Larry (1 October 2009). "Instrument Interfacing - The Great Paradox of LIMS?". LIMSfinder.com. http://www.limsfinder.com/BlogDetail.aspx?id=33851_0_2_0_C. Retrieved 8 May 2012.
- ↑ "LabCollector - Features in detail". AgileBio. http://labcollector.com/index.php/labcollector-on-pdahtml. Retrieved 8 May 2012.
- ↑ "Biometrics and DNA Laboratory Information Management and Analysis System Development". Future Technologies, Inc.. http://www.ftechi.com/dna_biometric.shtml. Retrieved 8 May 2012.
- ↑ "Modern web based Lims from 10 Euro cent/sample". Bytewize AB. http://www.bytewize.com/o3lims-xpress/prices/?lang=en. Retrieved 8 May 2012.