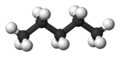Difference between revisions of "Pentane"
Shawndouglas (talk | contribs) (Created as needed) |
Shawndouglas (talk | contribs) m (1 revision imported) |
Revision as of 17:07, 27 December 2023
| |||
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Pentane[2] | |||
| Other names
Quintane;[1] Refrigerant-4-13-0
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| 969132 | |||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| DrugBank | |||
| ECHA InfoCard | 100.003.358 | ||
| EC Number |
| ||
| 1766 | |||
| MeSH | pentane | ||
PubChem CID
|
|||
| RTECS number |
| ||
| UNII | |||
| UN number | 1265 | ||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties[4] | |||
| C5H12 | |||
| Molar mass | 72.151 g·mol−1 | ||
| Appearance | Colourless liquid | ||
| Odor | Gasoline-like[3] | ||
| Density | 0.626 g/mL; 0.6262 g/mL (20 °C) | ||
| Melting point | −130.5 to −129.1 °C; −202.8 to −200.3 °F; 142.7 to 144.1 K | ||
| Boiling point | 35.9 to 36.3 °C; 96.5 to 97.3 °F; 309.0 to 309.4 K | ||
| 40 mg/L (20 °C) | |||
| log P | 3.255 | ||
| Vapor pressure | 57.90 kPa (20.0 °C) | ||
Henry's law
constant (kH) |
7.8 nmol Pa−1 kg−1 | ||
| Acidity (pKa) | ~45 | ||
| Basicity (pKb) | ~59 | ||
| UV-vis (λmax) | 200 nm | ||
| −63.05·10−6 cm3/mol | |||
Refractive index (nD)
|
1.358 | ||
| Viscosity | 0.240 mPa·s (at 20 °C) | ||
| Thermochemistry | |||
Heat capacity (C)
|
167.19 J K−1 mol−1 | ||
Std molar
entropy (S⦵298) |
263.47 J K−1 mol−1 | ||
Std enthalpy of
formation (ΔfH⦵298) |
−174.1–−172.9 kJ mol−1 | ||
Std enthalpy of
combustion (ΔcH⦵298) |
−3.5095–−3.5085 MJ mol−1 | ||
| Hazards | |||
| GHS labelling: | |||
   
| |||
| Danger | |||
| H225, H304, H336, H411 | |||
| P210, P261, P273, P301+P310, P331 | |||
| NFPA 704 (fire diamond) | |||
| Flash point | −49.0 °C (−56.2 °F; 224.2 K) | ||
| 260.0 °C (500.0 °F; 533.1 K) | |||
| Explosive limits | 1.5–7.8%[3] | ||
| Lethal dose or concentration (LD, LC): | |||
LD50 (median dose)
|
| ||
LC50 (median concentration)
|
130,000 mg/m3 (mouse, 30 min) 128,200 ppm (mouse, 37 min) 325,000 mg/m3 (mouse, 2 hr)[5] | ||
| NIOSH (US health exposure limits): | |||
PEL (Permissible)
|
TWA 1000 ppm (2950 mg/m3)[3] | ||
REL (Recommended)
|
TWA 120 ppm (350 mg/m3) C 610 ppm (1800 mg/m3) [15-minute][3] | ||
IDLH (Immediate danger)
|
1500 ppm[3] | ||
| Related compounds | |||
Related alkanes
|
|||
| Supplementary data page | |||
| Pentane (data page) | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Pentane is an organic compound with the formula C5H12—that is, an alkane with five carbon atoms. The term may refer to any of three structural isomers, or to a mixture of them: in the IUPAC nomenclature, however, pentane means exclusively the n-pentane isomer, in which case pentanes refers to a mixture of them; the other two are called isopentane (methylbutane) and neopentane (dimethylpropane). Cyclopentane is not an isomer of pentane because it has only 10 hydrogen atoms where pentane has 12.
Pentanes are components of some fuels and are employed as specialty solvents in the laboratory. Their properties are very similar to those of butanes and hexanes.
History
Normal pentane was discovered in 1862 by Carl Schorlemmer, who, while analyzing pyrolysis products of the cannel coal mined in Wigan, identified, separated by fractional distillation and studied a series of liquid hydrocarbons inert to nitric and sulfuric acids. The lightest of them, which he called hydride of amyl, had an empirical formula of C5H12, density of 0.636 at 17 °C and boiled between 39 and 40 °C.[6] In the next year he identified the same compound in the Pennsylvanian oil.[7] By 1872 he switched his nomenclature to the modern one, leading to it being called Pentane.[8]
Beyond Schorlemmer's initial work, scientists discovered that the molecular formula C5H12 could represent different structural arrangements, leading to the identification of isopentane and neopentane. This discovery contributed significantly to the understanding of isomerism and hydrocarbons in the 19th century. The high volatility and low boiling point of pentane made it useful as a solvent and in fuels. Its use expanded in the 1970s as a blowing agent for foams, replacing CFCs. The petroleum refining industry utilizes pentanes, particularly isopentane, to produce high-octane fuels.
Isomers
| Common name | normal pentane unbranched pentane n-pentane |
isopentane | neopentane |
| IUPAC name | pentane | 2-methylbutane | 2,2-dimethylpropane |
| Molecular diagram | 
|

|

|
| Skeletal diagram | 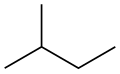
|

| |
| Melting point (°C)[9] | −129.8 | −159.9 | −16.6 |
| Boiling point (°C)[9] | 36.0 | 27.7 | 9.5 |
| Density (0 °C,kg/m3)[9] |
699 | 616 | 586 |
Industrial uses
Pentanes are some of the primary blowing agents used in the production of polystyrene foam and other foams. Usually, a mixture of n-, i-, and increasingly cyclopentane is used for this purpose.
Acid-catalyzed isomerization gives isopentane, which is used in producing high-octane fuels.[10]
Because of their low boiling points, low cost, and relative safety, pentanes are used as a working medium in geothermal power stations and organic Rankine cycles. It is also used in some blended refrigerants.
Pentanes are solvents in many ordinary products, e.g. in some pesticides.[11]
Laboratory use
Pentanes are relatively inexpensive and are the most volatile liquid alkanes at room temperature, so they are often used in the laboratory as solvents that can be conveniently and rapidly evaporated. However, because of their nonpolarity and lack of functionality, they dissolve only nonpolar and alkyl-rich compounds. Pentanes are miscible with most common nonpolar solvents such as chlorocarbons, aromatics, and ethers.
They are often used in liquid chromatography.
Physical properties
The boiling points of the pentane isomers range from about 9 to 36 °C. As is the case for other alkanes, the more thickly branched isomers tend to have lower boiling points.
The same tends to be true for the melting points of alkane isomers, and that of isopentane is 30 °C lower than that of n-pentane. However, the melting point of neopentane, the most heavily branched of the three, is 100 °C higher than that of isopentane. The anomalously high melting point of neopentane has been attributed to the tetrahedral molecules packing more closely in solid form; this explanation is contradicted by the fact that neopentane has a lower density than the other two isomers,[12] and the high melting point is actually caused by neopentane's significantly lower entropy of fusion.
The branched isomers are more stable (have lower heat of formation and heat of combustion) than n-pentane. The difference is 1.8 kcal/mol for isopentane, and 5 kcal/mol for neopentane.[13][14]
Rotation about two central single C-C bonds of n-pentane produces four different conformations.[15]
Reactions
Like other alkanes, pentanes are largely unreactive at standard room temperature and conditions - however, with sufficient activation energy (e.g., an open flame), they readily oxidize to form carbon dioxide and water:
- C5H12 + 8 O2 → 5 CO2 + 6 H2O + heat/energy
Like other alkanes, pentanes undergo free radical chlorination:
- C5H12 + Cl2 → C5H11Cl + HCl
Without zeolite catalysts, such reactions are unselective, so with n-pentane, the result is a mixture of the 1-, 2-, and 3-chloropentanes, as well as more highly chlorinated derivatives. Other radical halogenations can also occur.
Production and occurrence
Pentane is produced by fractional distillation of petroleum and purified by rectification (successive distillations).[16]
It occurs in alcoholic beverages and in hop oil.[16] It is a component of exhaled breath for some individuals. A degradation product of unsaturated fatty acids, its presence is associated with some diseases and cancers.[17]
Pentane is a relatively minor component of automobile gasoline, with its share varying within 1–6% in 1990s Sweden,[18] 2–13% in 1990s US[19] and 1–3% in the US in 2011.[20] At 62, its octane number (both RON and MON) is quite low.[21]
References
- ^ Hofmann, August Wilhelm Von (1 January 1867). "I. On the action of trichloride of phosphorus on the salts of the aromatic monamines". Proceedings of the Royal Society of London. 15: 54–62. doi:10.1098/rspl.1866.0018. S2CID 98496840.
- ^ International Union of Pure and Applied Chemistry (2014). Nomenclature of Organic Chemistry: IUPAC Recommendations and Preferred Names 2013. The Royal Society of Chemistry. p. 59. doi:10.1039/9781849733069. ISBN 978-0-85404-182-4.
- ^ a b c d e NIOSH Pocket Guide to Chemical Hazards. "#0486". National Institute for Occupational Safety and Health (NIOSH).
- ^ Record of n-Pentane in the GESTIS Substance Database of the Institute for Occupational Safety and Health, accessed on 19 April 2011.
- ^ "n-Pentane". Immediately Dangerous to Life or Health Concentrations. National Institute for Occupational Safety and Health.
- ^ Schorlemmer, C. (1862). "On the hydrides of the alcohol-radicles existing in the products of the destructive distillation of cannel coal". Journal of the Chemical Society. 15: 419–427. doi:10.1039/JS8621500419. ISSN 0368-1769.
- ^ Proceedings of the Literary and Philosophical Society of Manchester. 1864.
- ^ Schorlemmer, Carl (1872). "On the normal paraffins". Philosophical Transactions of the Royal Society of London. 162: 111–123. doi:10.1098/rstl.1872.0007.
- ^ a b c Wei, James (1999). "Molecular Symmetry, Rotational Entropy, and Elevated Melting Points". Industrial & Engineering Chemistry Research. 38 (12): 5019–5027. doi:10.1021/ie990588m.
- ^ Karl Griesbaum; Arno Behr; Dieter Biedenkapp; Heinz-Werner Voges; Dorothea Garbe; Christian Paetz; Gerd Collin; Dieter Mayer; Hartmut Höke (2002). "Hydrocarbons". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a13_227. ISBN 978-3-527-30673-2.
- ^ Milne, G. W. A., ed. (2005). Gardner's Commercially Important Chemicals: Synonyms, Trade Names, and Properties. Hoboken, New Jersey: John Wiley & Sons, Inc. p. 477. ISBN 978-0-471-73518-2.
- ^ Wei, James (1999). "Molecular Symmetry, Rotational Entropy, and Elevated Melting Points". Industrial & Engineering Chemistry Research. 38 (12). American Chemical Society (ACS): 5019–5027. doi:10.1021/ie990588m. ISSN 0888-5885.
- ^ From the values listed at Standard enthalpy change of formation (data table).
- ^ Good, W.D (1970). "The enthalpies of combustion and formation of the isomeric pentanes". The Journal of Chemical Thermodynamics. 2 (2). Elsevier BV: 237–244. Bibcode:1970JChTh...2..237G. doi:10.1016/0021-9614(70)90088-1. ISSN 0021-9614.
- ^ Roman M. Balabin (2009). "Enthalpy Difference between Conformations of Normal Alkanes: Raman Spectroscopy Study of n-Pentane and n-Butane". J. Phys. Chem. A. 113 (6): 1012–9. Bibcode:2009JPCA..113.1012B. doi:10.1021/jp809639s. PMID 19152252.
- ^ a b "Pentane". PubChem. Retrieved 2023-06-29.
- ^ Phillips, Michael; Herrera, Jolanta; Krishnan, Sunithi; Zain, Mooena; Greenberg, Joel; Cataneo, Renee N. (1999). "Variation in volatile organic compounds in the breath of normal humans". Journal of Chromatography B: Biomedical Sciences and Applications. 729 (1–2): 75–88. doi:10.1016/S0378-4347(99)00127-9. PMID 10410929.
- ^ Östermark, Ulf; Petersson, Göran (1992-09-01). "Assessment of hydrocarbons in vapours of conventional and alkylate-based petrol" (PDF). Chemosphere. 25 (6): 763–768. Bibcode:1992Chmsp..25..763O. doi:10.1016/0045-6535(92)90066-Z. ISSN 0045-6535.
- ^ Doskey, Paul V.; Porter, Joseph A.; Scheff, Peter A. (November 1992). "Source Fingerprints for Volatile Non-Methane Hydrocarbons". Journal of the Air & Waste Management Association. 42 (11): 1437–1445. Bibcode:1992JAWMA..42.1437D. doi:10.1080/10473289.1992.10467090. ISSN 1047-3289.
- ^ "Hydrocarbon Composition of Gasoline Vapor Emissions from Enclosed Fuel Tanks". nepis.epa.gov. United States Environmental Protection Agency. 2011.
- ^ Scherzer, Julius (1990). Octane-Enhancing Zeolitic FCC Catalysts: Scientific and Technical Aspects. CRC Press. p. 9. ISBN 978-0-8247-8399-0.
External links
- International Chemical Safety Card 0534 at ILO.org
- NIOSH Pocket Guide to Chemical Hazards at CDC.gov
- Phytochemical data for pentane at Ars-grin.gov
Notes
This article is a direct transclusion of the Wikipedia article and therefore may not meet the same editing standards as CannabisQAwiki.