Difference between revisions of "Main Page/Featured article of the week/2023"
Shawndouglas (talk | contribs) (Added last week's article of the week) |
Shawndouglas (talk | contribs) (Added last week's article of the week) |
||
| Line 17: | Line 17: | ||
<!-- Below this line begin pasting previous news --> | <!-- Below this line begin pasting previous news --> | ||
<h2 style="font-size:105%; font-weight:bold; text-align:left; color:#000; padding:0.2em 0.4em; width:50%;">Featured article of the week: February 13–19:</h2> | <h2 style="font-size:105%; font-weight:bold; text-align:left; color:#000; padding:0.2em 0.4em; width:50%;">Featured article of the week: February 20–27:</h2> | ||
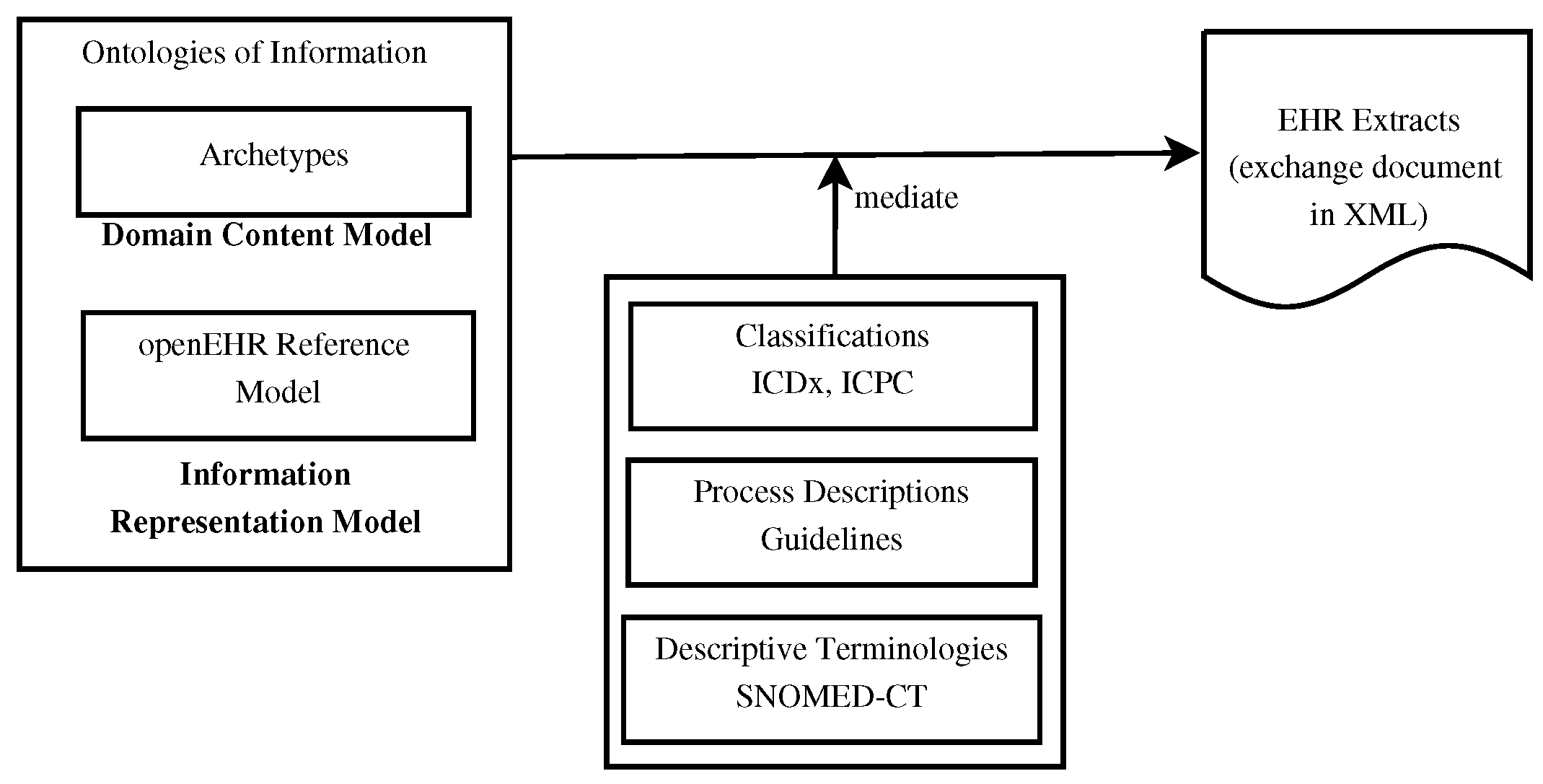
<div style="float: left; margin: 0.5em 0.9em 0.4em 0em;">[[File:Fig2 Yoon LabAniRes22 38.png|240px]]</div> | |||
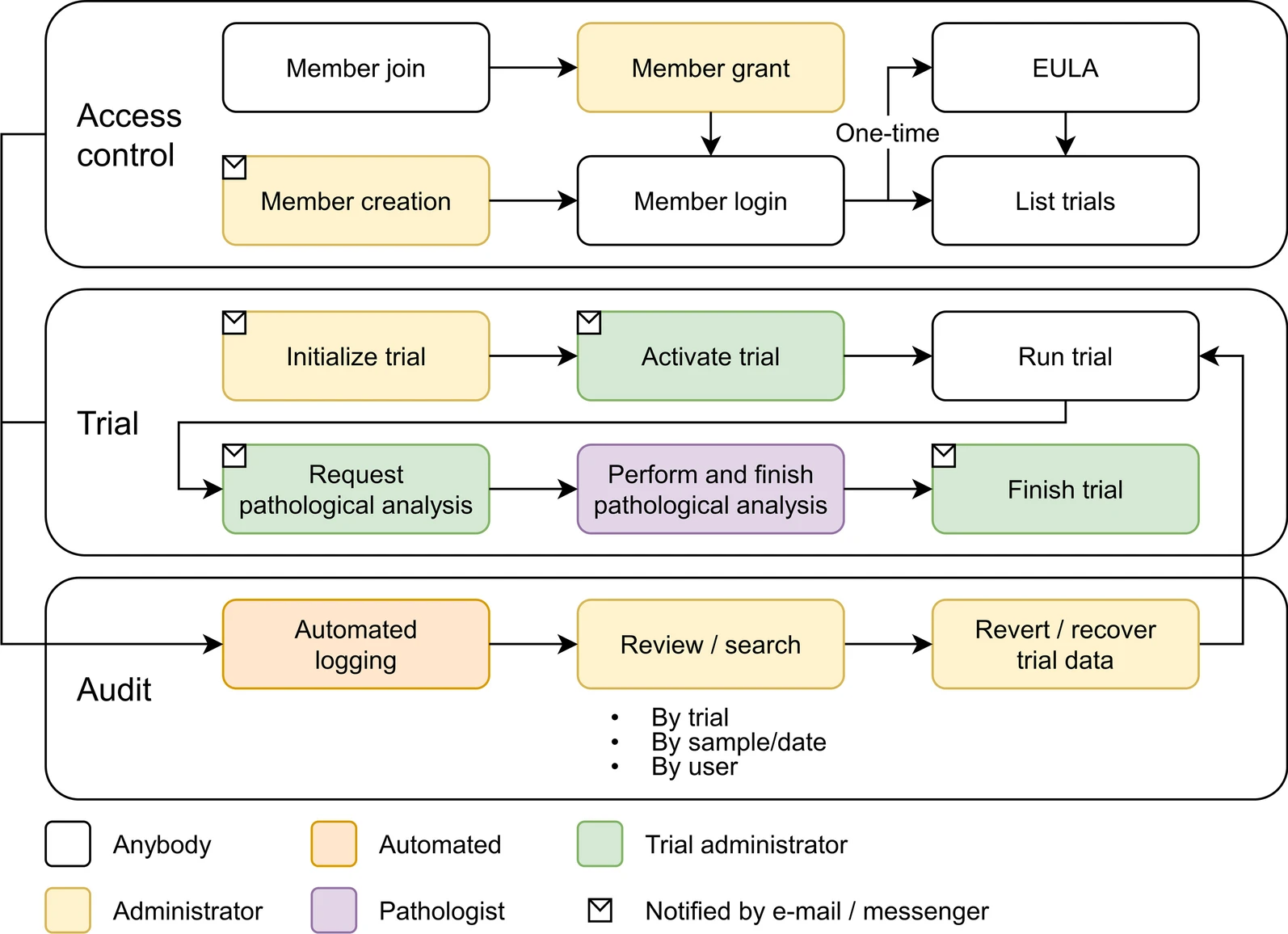
'''"[[Journal:Laboratory information management system for COVID-19 non-clinical efficacy trial data|Laboratory information management system for COVID-19 non-clinical efficacy trial data]]"''' | |||
As the number of large-scale research studies involving multiple organizations producing data has steadily increased, an integrated system for a common interoperable data format is needed. For example, in response to the [[coronavirus disease 2019]] (COVID-19) [[pandemic]], a number of global efforts are underway to develop vaccines and therapeutics. We are therefore observing an explosion in the proliferation of COVID-19 data, and interoperability is highly requested in multiple institutions participating simultaneously in COVID-19 pandemic research. In this study, a [[laboratory information management system]] (LIMS) has been adopted to systemically manage, via web interface, various COVID-19 non-clinical trial data—including mortality, clinical signs, body weight, body temperature, organ weights, viral titer (viral replication and viral RNA), and multi-organ [[histopathology]]—from multiple institutions ... ('''[[Journal:Laboratory information management system for COVID-19 non-clinical efficacy trial data|Full article...]]''')<br /> | |||
|- | |||
|<br /><h2 style="font-size:105%; font-weight:bold; text-align:left; color:#000; padding:0.2em 0.4em; width:50%;">Featured article of the week: February 13–19:</h2> | |||
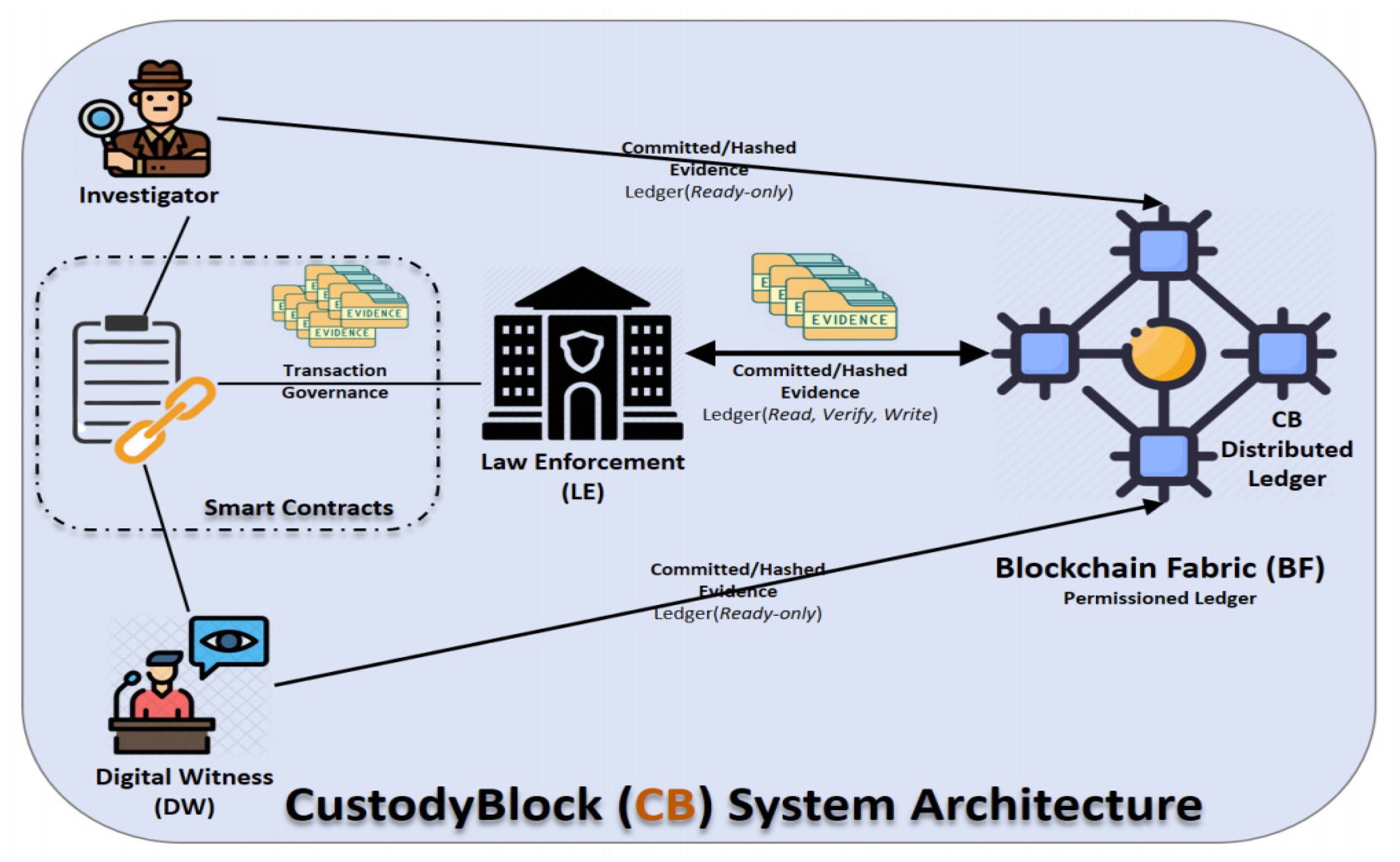
<div style="float: left; margin: 0.5em 0.9em 0.4em 0em;">[[File:Fig1 AbuHalimeh FrontBigData2022 5.jpg|240px]]</div> | <div style="float: left; margin: 0.5em 0.9em 0.4em 0em;">[[File:Fig1 AbuHalimeh FrontBigData2022 5.jpg|240px]]</div> | ||
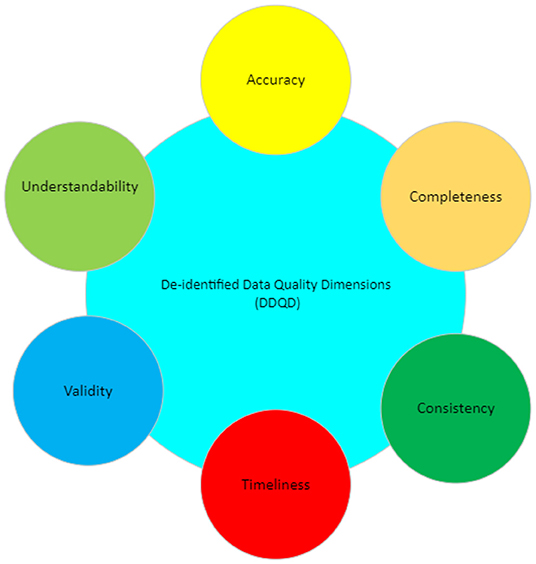
'''"[[Journal:Improving data quality in clinical research informatics tools|Improving data quality in clinical research informatics tools]]"''' | '''"[[Journal:Improving data quality in clinical research informatics tools|Improving data quality in clinical research informatics tools]]"''' | ||
Maintaining [[data quality]] is a fundamental requirement for any successful and long-term [[Information management|data management]] project. Providing high-quality, reliable, and statistically sound data is a primary goal for [[wikipedia:Clinical research|clinical research]] [[Informatics (academic field)|informatics]]. In addition, effective data governance and management are essential to ensuring accurate data counts, reports, and validation. As a crucial step of the clinical research process, it is important to establish and maintain organization-wide standards for data quality management to ensure consistency across all systems designed primarily for cohort identification ... ('''[[Journal:Improving data quality in clinical research informatics tools|Full article...]]''')<br /> | Maintaining [[data quality]] is a fundamental requirement for any successful and long-term [[Information management|data management]] project. Providing high-quality, reliable, and statistically sound data is a primary goal for [[wikipedia:Clinical research|clinical research]] [[Informatics (academic field)|informatics]]. In addition, effective data governance and management are essential to ensuring accurate data counts, reports, and validation. As a crucial step of the clinical research process, it is important to establish and maintain organization-wide standards for data quality management to ensure consistency across all systems designed primarily for cohort identification ... ('''[[Journal:Improving data quality in clinical research informatics tools|Full article...]]''')<br /> | ||
|- | |||
|<br /><h2 style="font-size:105%; font-weight:bold; text-align:left; color:#000; padding:0.2em 0.4em; width:50%;">Featured article of the week: February 6–12:</h2> | |<br /><h2 style="font-size:105%; font-weight:bold; text-align:left; color:#000; padding:0.2em 0.4em; width:50%;">Featured article of the week: February 6–12:</h2> | ||
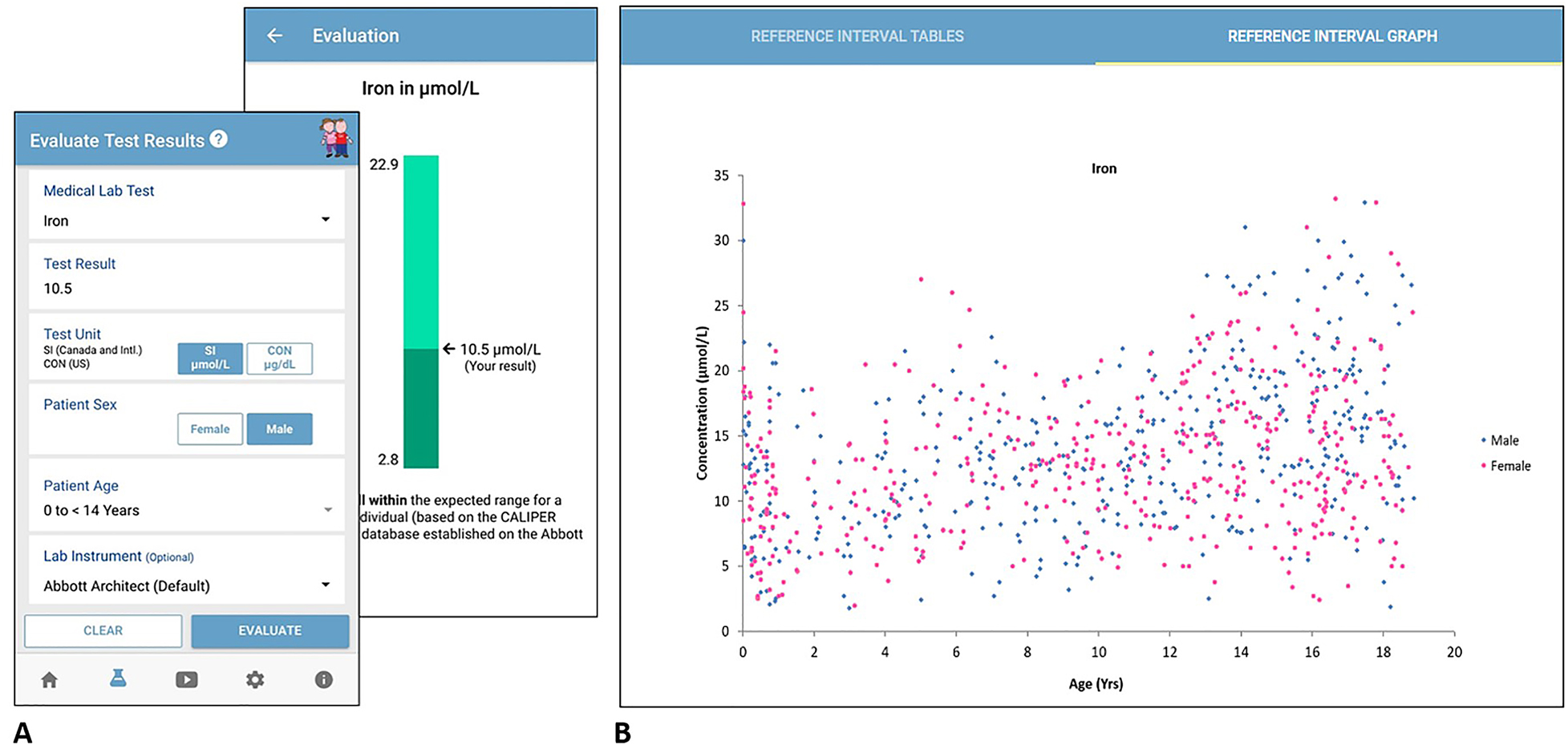
<div style="float: left; margin: 0.5em 0.9em 0.4em 0em;">[[File:Fig1 Bohn JofLabMed2021 45-6.jpg|240px]]</div> | <div style="float: left; margin: 0.5em 0.9em 0.4em 0em;">[[File:Fig1 Bohn JofLabMed2021 45-6.jpg|240px]]</div> | ||
Revision as of 16:21, 27 February 2023
|
|
If you're looking for other "Article of the Week" archives: 2014 - 2015 - 2016 - 2017 - 2018 - 2019 - 2020 - 2021 - 2022 - 2023 |
Featured article of the week archive - 2023
Welcome to the LIMSwiki 2023 archive for the Featured Article of the Week.
Featured article of the week: February 20–27:"Laboratory information management system for COVID-19 non-clinical efficacy trial data" As the number of large-scale research studies involving multiple organizations producing data has steadily increased, an integrated system for a common interoperable data format is needed. For example, in response to the coronavirus disease 2019 (COVID-19) pandemic, a number of global efforts are underway to develop vaccines and therapeutics. We are therefore observing an explosion in the proliferation of COVID-19 data, and interoperability is highly requested in multiple institutions participating simultaneously in COVID-19 pandemic research. In this study, a laboratory information management system (LIMS) has been adopted to systemically manage, via web interface, various COVID-19 non-clinical trial data—including mortality, clinical signs, body weight, body temperature, organ weights, viral titer (viral replication and viral RNA), and multi-organ histopathology—from multiple institutions ... (Full article...)
|