Difference between revisions of "User:Shawndouglas/sandbox/sublevel15"
Shawndouglas (talk | contribs) (Replaced content with "<div class="nonumtoc">__TOC__</div> {{ombox | type = notice | style = width: 960px; | text = This is sublevel15 of my sandbox, where I play with features and...") Tag: Replaced |
Shawndouglas (talk | contribs) Tag: Reverted |
||
| Line 7: | Line 7: | ||
==Sandbox begins below== | ==Sandbox begins below== | ||
<!--{{LIMS Selection Guide for Manufacturing Quality Control/Blank LIMSpec template for manufacturing labs/Introduction and methodology}}//--> | |||
==A1. Introduction and methodology== | |||
{{LIMSpec/Introduction and methodology}} | |||
<!--{{LIMS Selection Guide for Manufacturing Quality Control/Blank LIMSpec template for manufacturing labs/Primary laboratory workflow}}//--> | |||
__NOTOC__ | |||
==A2. Primary laboratory workflow== | |||
'''Note: These categories cover what [[ASTM E1578|ASTM E1578-18]] considers to be the core of your typical laboratory workflow, from sample/specimen receipt and sampling procedures, to analysis, review, and reporting.''' | |||
===1. Sample and experiment registration=== | |||
{{LIMSpec/Sample and experiment registration}} | |||
===2. Sample management=== | |||
{{LIMSpec/Sample management}} | |||
===3. Core laboratory testing and experiments=== | |||
{{LIMSpec/Core laboratory testing and experiments}} | |||
===4. Results review and verification=== | |||
{{LIMSpec/Results review and verification}} | |||
===5. Sample, experiment, and study approval and verification=== | |||
{{LIMSpec/Sample, experiment, and study approval and verification}} | |||
===6. Reporting=== | |||
{{LIMSpec/Reporting}} | |||
<!--{{LIMS Selection Guide for Manufacturing Quality Control/Blank LIMSpec template for manufacturing labs/Maintaining laboratory workflow and operations}}//--> | |||
__NOTOC__ | |||
==A3. Maintaining laboratory workflow and operations== | |||
'''Note: These categories cover what [[ASTM E1578|ASTM E1578-18]] considers to be the extended functions that support the previously described core laboratory workflow, from scheduling, batch/lot management, and instrument management to standard/reagent management and investigation management.''' | |||
===7. Document and records management=== | |||
{{LIMSpec/Document management}} | |||
===8. Resource management=== | |||
{{LIMSpec/Resource management}} | |||
===9. Compliance management=== | |||
{{LIMSpec/Compliance management}} | |||
===10. Instrument and equipment management=== | |||
{{LIMSpec/Instrument and equipment management}} | |||
===11. Batch and lot management=== | |||
{{LIMSpec/Batch and lot management}} | |||
===12. Scheduled event management=== | |||
{{LIMSpec/Scheduled event management}} | |||
===13. Instrument data capture and control=== | |||
{{LIMSpec/Instrument data capture and control}} | |||
===14. Standard and reagent management=== | |||
{{LIMSpec/Standard and reagent management}} | |||
===15. Inventory management=== | |||
{{LIMSpec/Inventory management}} | |||
===16. Investigation and quality management=== | |||
{{LIMSpec/Investigation and quality management}} | |||
<!--{{LIMS Selection Guide for Manufacturing Quality Control/Blank LIMSpec template for manufacturing labs/Specialty laboratory functions}}//--> | |||
__NOTOC__ | |||
==A4. Specialty laboratory functions== | |||
'''Note: These categories cover the specialty requirements that come with working in specific industries such as agriculture, pharmaceutical production, and [[forensic science]]. You'll likely notice that most of the content here isn't covered by [[ASTM E1578|ASTM E1578-18]].''' | |||
===17. Production management=== | |||
{{LIMSpec/Production management}} | |||
===18. Statistical trending and control charts=== | |||
{{LIMSpec/Statistical trending and control charts}} | |||
===19. Agriculture and food data management=== | |||
{{LIMSpec/Agriculture and food data management}} | |||
===20. Environmental data management=== | |||
Not relevant to manufacturing labs. | |||
===21. Forensic case and data management=== | |||
Not relevant to manufacturing labs. | |||
===22. Clinical and public health data management=== | |||
Not relevant to manufacturing labs. | |||
===23. Veterinary data management=== | |||
Not relevant to manufacturing labs. | |||
===24. Scientific data management=== | |||
{{LIMSpec/Scientific data management}} | |||
===25. Health information technology=== | |||
Not relevant to manufacturing labs. | |||
<!--{{LIMS Selection Guide for Manufacturing Quality Control/Blank LIMSpec template for manufacturing labs/Technology and performance improvements}}//--> | |||
__NOTOC__ | |||
==A5. Technology and performance improvements== | |||
'''Note: These categories cover what [[ASTM E1578|ASTM E1578-18]] considers to be the extended functions that support core laboratory workflow, but in this case the dominant theme is additional technology that supports workflow, as well as requirements that improve overall laboratory performance.''' | |||
===26. Instrument data systems functions=== | |||
{{LIMSpec/Instrument data systems functions}} | |||
===27. Systems integration=== | |||
{{LIMSpec/Systems integration}} | |||
===28. Laboratory scheduling and capacity planning=== | |||
{{LIMSpec/Laboratory scheduling and capacity planning}} | |||
===29. Lean laboratory and continuous improvement=== | |||
{{LIMSpec/Lean laboratory and continuous improvement}} | |||
===30. Artificial intelligence and smart systems=== | |||
{{LIMSpec/Artificial intelligence and smart systems}} | |||
<!--{{LIMS Selection Guide for Manufacturing Quality Control/Blank LIMSpec template for manufacturing labs/Security and integrity of systems and operations}}//--> | |||
__NOTOC__ | |||
==A6. Security and integrity of systems and operations== | |||
'''Note: These categories cover what [[ASTM E1578|ASTM E1578-18]] largely considers to be "platform and administration support functions." Notably, most of the requirements here have something to do with ensuring the security and integrity of not only the system and its functions but also the data that it houses and modifies.''' | |||
===31. Data integrity=== | |||
{{LIMSpec/Data integrity}} | |||
===32. Configuration management=== | |||
{{LIMSpec/Configuration management}} | |||
===33. System validation and commission=== | |||
{{LIMSpec/System validation and commission}} | |||
===34. System administration=== | |||
{{LIMSpec/System administration}} | |||
===35. Cybersecurity=== | |||
{{LIMSpec/Cybersecurity}} | |||
===36. Information privacy=== | |||
{{LIMSpec/Information privacy}} | |||
<!--{{LIMS Selection Guide for Manufacturing Quality Control/Blank LIMSpec template for manufacturing labs/Putting LIMSpec to Use}}//--> | |||
__NOTOC__ | |||
==A7. Putting those requirements to practical use and caveats== | |||
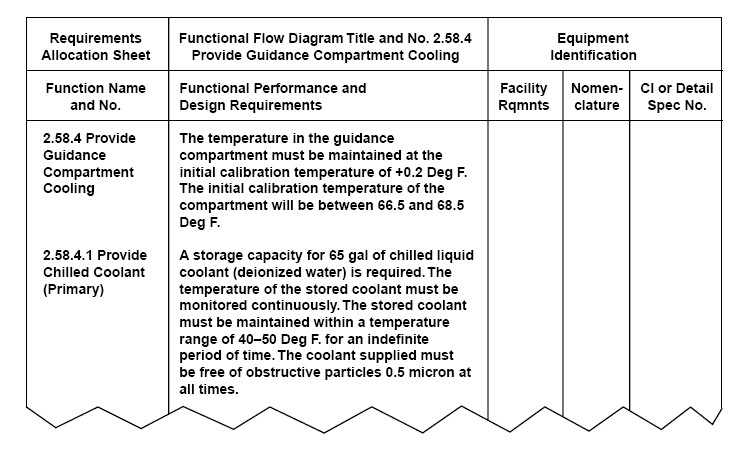
[[File:Requirements Allocation Sheet.jpg|600px|right]]The LIMSpec covered [[laboratory informatics]] requirements organized into five broad categories, which are heavily influenced by the functional requirements checklist and Figure 3 of [[ASTM E1578|ASTM E1578-18]] ''Standard Guide for Laboratory Informatics''. However, the requirements listed prior are all based on not just the ASTM E1578 standard but also a wide variety of other standards, regulations, guidance documents, and standardized procedures (hereon referred to as "sources"). That ultimately means a foundational reasoning is provided for each requirement, not necessarily a "just because I want it" reasoning. As foundational requirements, this LIMSpec should thus operate as an excellent starting point for building your own software requirements specification or for researching the best laboratory informatics solution for your [[laboratory]]. | |||
===Software developer considerations=== | |||
What does that mean for you? How can you best use this document? If you're a software developer for the laboratory industry, many of the sources referenced in these requirements should already be familiar to you. However, some of them may not be, and you'll probably want to at least familiarize yourself with them. Additionally, if you're developing a generic [[laboratory information management system]] (LIMS) or some other informatics solution, not tailored to a particular industry, most everything in chapters two, three, five, and six should largely be applicable to what you're doing with your commercial off-the-shelf (COTS) software solution. Definitely review the requirements items listed there and make sure the most important ones are part of your own software requirements specification. If the software solution you're developing is tailored to a particular industry (e.g., clinical or public health, pharmaceutical development, or heavy metals testing), you'll also want to examine chapter four. If you don't see many requirements for your industry listed (see the "Caveats" section later), you'll probably have additional research to conduct to see what additional sources will affect how you develop the functional and, particularly, non-functional requirements. | |||
===Buyer considerations=== | |||
If you're a potential buyer of a laboratory informatics solution, this LIMSpec is also useful to you. Perhaps you know a bit about your laboratory's workflow and a few of the regulations and standards that influence how that workflow is conducted, but you're not entirely informed. Reviewing the five broad categories of requirements may be necessary to help further inform you regarding what's vital in regards to what a laboratory informatics solution should be capable of. Additionally, you can then use these requirements as a base for your laboratory's own requirements list. Using the categories and their subdivisions, you can then add those requirements that are unique to your laboratory and industry that are not sufficiently covered by the LIMSpec requirements. As you review the various options available to you and narrow down your search, your own list of requirements can be used as both as a personal checklist and as a requirements list you hand over to the vendor you query. | |||
====Software vendor selection==== | |||
That said, the requirements you hand off to the vendor should be discussed a bit more. Software vendor selection can at times be a tedious yet necessary process, one which requires careful planning and best practices. This topic has been written about by both software developers and end users alike, and their experiences should play a role in how you select a vendor. What follows is bullet-pointed advice as offered by some of those developers and end users.<ref name="PearceSoftware16">{{cite web |url=https://blog.montrium.com/blog/software-vendor-selection-defining-your-requirements |title=Software Vendor Selection: How to Define Your Requirements |work=Montrium Blog |author=Pearce, O. |publisher=Montrium, Inc |date=21 June 2016 |accessdate=07 December 2022}}</ref><ref name="PearceSoftware16-2">{{cite web |url=https://blog.montrium.com/blog/software-vendor-selection-finding-the-right-vendor |title=Software Vendor Selection: Finding the Right Vendor |work=Montrium Blog |author=Pearce, O. |publisher=Montrium, Inc |date=23 June 2016 |accessdate=07 December 2022}}</ref><ref name="PearceSoftware16-3">{{cite web |url=https://blog.montrium.com/blog/software-vendor-selection-conducting-demonstrations |title=Software Vendor Selection: The Pitfalls and Successes of Vendor Demos |work=Montrium Blog |author=Pearce, O. |publisher=Montrium, Inc |date=28 June 2016 |accessdate=07 December 2022}}</ref><ref name="PearceSoftware16-4">{{cite web |url=https://blog.montrium.com/blog/software-vendor-selection-requesting-proposals-quotes |title=Software Vendor Selection: Requesting Proposals & Quotes |work=Montrium Blog |author=Pearce, O. |publisher=Montrium, Inc |date=05 July 2016 |accessdate=07 December 2022}}</ref><ref name="PersaudBusiness16">{{cite web |url=https://www.selecthub.com/miscellaneous/technology-selection/business-requirements-gathering-enterprise-software-selection/ |title=Business Requirements Gathering for Enterprise Software Selection |author=Persaud, D. |work=SelectHub Blog |publisher=Abuyo, Inc |date=04 February 2016 |accessdate=07 December 2022}}</ref><ref name="LichtenbergerSix12">{{cite web |url=https://blog.itil.org/2012/07/six-steps-for-a-successful-vendor-selection/ |title=Six Steps for a Successful Vendor Selection |author=Lichtenberger, A. |work=ITIL.org |date=23 July 2012 |accessdate=07 December 2022}}</ref><ref name="PoonInsider15">{{cite web |url=https://www.genologics.com/blog/insiders-guide-to-lims-selection/ |title=Insider’s Guide to LIMS Selection |author=Poon, L. |work=Genologics Blog |publisher=GenoLogics Life Sciences Software Inc |date=29 May 2015 |accessdate=20 September 2019}}{{Dead link|date=December 2022}}</ref><ref name="BenchlingHowTo">{{cite web |url=https://benchling.com/static/docs/resources/eln-for-biology-rnd.pdf |title=How to Select an ELN for Biology R&D |publisher=Benchling, Inc |accessdate=07 December 2022}}</ref> | |||
* Have a clear business case and build your business needs into your laboratory's requirements. | |||
* Be mindful of how detailed you get with your own business-based requirements and what you initially hand off to a vendor. If you're too specific with too many requirements, you may have trouble finding a vendor that matches up. Start with the essentials that involve your laboratory's processes, regulations, integrations, reporting, service needs, etc. As this LIMSpec is foundation-based, you have a good starting point in that regard. You can always get more detailed with requirements as you narrow down vendors. | |||
* As discussed briefly in the introduction, you'll need to prioritize your needs somewhere between "critical" and "nice to have." The LIMSpec's requirements are largely critical for most purposes and can be marked as such. The requirements you add will have to be prioritized more carefully. | |||
* You'll also want to perform some informal third-party information gathering about the vendors. Are reviews of the vendors trustworthy? Have peers had any interactions and success with the vendor? Does the vendor have the ability to scale to meet your needs? | |||
* Schedule demonstrations of programs that seem like strong initial candidates. Make sure there is a question and answer session afterwards, and perform a post-demo evaluation. | |||
* A formal request for proposal (RFP) may or may not be necessary, depending on the level of information you acquire prior. However, formally requesting pricing and clarification of maintenance and additional service costs is useful. Just don't let price be the only thing that guides you. | |||
* Consider some of the intangibles. Does the vendor genuinely seem interested in your business and its needs? Do they communicate well and promptly? Do they seem flexible and able to accommodate a few special case requirements? | |||
* Be sure to consider future needs as you anticipate potential laboratory expansion. | |||
* Don't be afraid to choose a consultant to help you with the vendor selection process. | |||
===Caveats=== | |||
First, note that this LIMSpec is still an evolving entity. Standards change. Regulations change. Procedures also change with such standards and regulations. That means that as those foundational characteristics shift, this set of requirements will have to also evolve. As such, do your homework and don't take everything you see here as fixed law. If you're responsible for investigating and/or purchasing a laboratory informatics system, be sure you have at least some familiarity with the primary industry your laboratory serves, and by extension the regulations and standards that affect it. | |||
Second, the number of industry-specific applications of laboratory informatics software continues to grow, and with it also the regulations and standards that affect those specialty laboratories. As such, some industry-specific requirements may have been missed for lack of or too expensive public-facing sources. As mentioned with the first caveat, this version of LIMSpec is evolving, and as industry experts and researchers are able to provide additional feedback on this document, it will surely grow with more relevant sources. In other words, don't consider this complete, particularly if you're in a specialized laboratory industry. You may have to add more items based on you industry knowledge and insights. | |||
<!--{{LIMS Selection Guide for Manufacturing Quality Control/Blank LIMSpec template for manufacturing labs/LIMSpec in Microsoft Word format}}//--> | |||
==A8. LIMSpec in Microsoft Word format== | |||
Microsoft Excel is often used as a tool to document requirements specifications. However, one downside to Microsoft Excel is its inability to handle multiple hyperlinks in the same cell. If you've looked over the LIMSpec in Appendix 1, you've likely noticed there are multiple hyperlinks to regulations, specifications, and guidance documents in the first column of the tables. Translating these wiki-based documents to Excel makes for a challenge when trying to maintain those hyperlinks. As they add value to not only your laboratory's requirements research but also to vendors' understanding of the sources for your requirements, it was decided the hyperlinks should be maintained in any portable version. As such, a Microsoft Word version was created. | |||
You can download a copy of the Microsoft Word version of LIMSpec from LIMSwiki by going to [[File:LIMSpec for Manufacturing Labs v1.0.docx|this file page]], right-clicking the URL under the white box, and selecting "Save link as..." (Alternatively, you can just click the link, open the file, and then save it.) A compromise was made between keeping the hyperlinks in the first column readable and leaving enough room in the third column for a vendor to provide a response. This response space admittedly may be a limiting factor for vendors wanting to include screenshots. If this situation arises, you may encourage the vendor to select the entire first column and delete it, then widening the response column. | |||
Note that this downloadable version of LIMSpec is released under the same licensing terms as this guide. Please see the first paragraph of the download for more details. | |||
==References== | |||
{{Reflist|colwidth=30em}} | |||
Revision as of 18:29, 22 March 2023
|
|
This is sublevel15 of my sandbox, where I play with features and test MediaWiki code. If you wish to leave a comment for me, please see my discussion page instead. |
Sandbox begins below
A1. Introduction and methodology
Introduction
Merriam-Webster defines a "specification" as "a detailed precise presentation of something or of a plan or proposal for something."[1] In other words, an existing or theoretical product, concept, or idea is presented in detail for a particular audience. In a broad sense, detailing the specifics about a project, concept, or idea to others is just common sense. This applies just as well to the world of software development, where a software requirements specification is essential for preventing the second most commonly cited reason for project failure: poor requirements management.[2]
In fact, the ISO/IEC/IEEE 29148:2018 standard (a conglomeration of what was formerly IEEE 830 and other standards) is in place to help specify "the required processes implemented in the engineering activities that result in requirements for systems and software products" and provides guidelines for how to apply those requirements.[3] The standard describes the characteristics that make up quality software requirement development, including aspects such as[4]:
- correctly describing system behavior;
- effectively removing ambiguity from the language used;
- completely covering the system behavior and features;
- accurately prioritizing and ranking the requirements; and
- unequivocally ensuring the requirements are testable, modifiable, and traceable.
A requirement typically comes in the form of a statement that begins with "the system/user/vendor shall/should ..." and focuses on a provided service, reaction to input, or expected behavior in a given situation. The statement may be abstract (high-level) or specific and detailed to a precise function. The statement may also be of a functional nature, describing functionality or services in detail, or of a non-functional nature, describing the constraints of a given functionality or service and how it's rendered. An example of a functional software requirement could be "the user shall be able to query either all of the initial set of databases or select a subset from it." This statement describes specific functionality the system should have. On the other hand, a non-functional requirement, for example, may state "the system's query tool shall conform to the ABC 123-2014 standard." The statement describes a constraint placed upon the system's query functionality. Once compiled, a set of requirements can serve not only to strengthen the software requirements specification, but the requirements set can also be used for bidding on a contract or serve as the basis for a specific contract that is being finalized.[5]
Over the years, a wide variety of companies, consultants, and researchers have compiled public and private software requirements specifications for laboratory informatics systems. These compiled lists of requirements for how a given laboratory informatics solution should be developed, delivered, and maintained have changed as technology and user demand have evolved. Often times, these requirements documents turn into a mix of "wishlist" requirements from potential and active clients, as well as regulation-mandated requirements. The wishlist items aren't necessarily ignored by developers, but they do in fact have to be prioritized as "nice to have" or "essential to system operation," or something in between.[6][7][8] While this reasonable mix of requirements has served informatics software developers well[9], sometimes a fresh approach is required.
What follows is an attempt to look less at the wishlists of laboratories and more directly at what requirements current regulatory schemes, industry standards, and organizational guidelines place on the ever-evolving array of laboratory informatics systems being developed today. What does the United States' 21 CFR Part 11 have to say about how your laboratory information management system (LIMS), laboratory information system (LIS), electronic laboratory notebook (ELN), and other systems operate? What does the European Union's Annex 11 dictate in those same regards? The following five chapters list those requirements, supported by one or more regulations, standards, and guidelines. The final chapter discusses how to best put this requirements specification to use.
Methodology
At its core, this LIMSpec—which has seen several iterations over the years—is rooted in ASTM E1578-18 Standard Guide for Laboratory Informatics. The latest version was released in 2018, which includes an updated Laboratory Informatics Functional Requirements checklist in the appendix. That list of requirements "covers functionality common to the various laboratory informatics systems discussed throughout [the] guide as well as requirements recommended as part of [the] guide." It goes on to state that the checklist "is an example of typical requirements that can be used to guide the purchase, upgrade, or development of a laboratory informatics system," though it is certainly "not meant to be exhaustive."
This LIMSpec borrows from that requirements checklist and then adds more to it from a wide variety of sources. An attempt has been made to find the most relevant regulations, standards, and guidance that shape how a compliant laboratory informatics system is developed and maintained. However, this should definitely be considered a work in progress, with more to be added with additional public and private comment on missing sources.
That said, this fourth revision (December 2022) taps into more than 130 resources, including the following:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Each requirement statement has at least one linked regulation, standard, or guidance item. In some cases, the standards covered are proprietary. In those cases, the standard was either purchased for review or heavily researched using supporting documentation, and the link goes to the acquisition page for the standard. In other cases, some sources have been intentionally omitted. For example, the AOAC International Official Methods of Analysis and Guidelines for Laboratories Performing Microbiological and Chemical Analyses of Food, Dietary Supplements, and Pharmaceuticals are both proprietary and more or less prohibitively expensive. In other cases, such as with the U.S. Food Emergency Response Network and Laboratory Response Network, they simply don't make their standardized procedures open to the public and thus can't be included.
A2. Primary laboratory workflow
Note: These categories cover what ASTM E1578-18 considers to be the core of your typical laboratory workflow, from sample/specimen receipt and sampling procedures, to analysis, review, and reporting.
1. Sample and experiment registration
2. Sample management
| ||||||||||||||||||||||||||||
3. Core laboratory testing and experiments
| ||||||||||||||||||||||||||||||||
4. Results review and verification
| ||||||||||||||||||
5. Sample, experiment, and study approval and verification
| ||||||||||
6. Reporting
| ||||||||||||||||||||||
A3. Maintaining laboratory workflow and operations
Note: These categories cover what ASTM E1578-18 considers to be the extended functions that support the previously described core laboratory workflow, from scheduling, batch/lot management, and instrument management to standard/reagent management and investigation management.
7. Document and records management
| ||||||||||||||||||||||||||
8. Resource management
| ||||||||||||||||||||
9. Compliance management
| ||||||||||||||||||
10. Instrument and equipment management
| ||||||||||||||||||||||||||||||||||
11. Batch and lot management
| ||||||||||||||
12. Scheduled event management
| ||||||||||||||
13. Instrument data capture and control
| ||||||||||||||||||||
14. Standard and reagent management
| ||||||||||||||
15. Inventory management
| ||||||||||||||||||||
16. Investigation and quality management
| ||||||||||||||||||||||
A4. Specialty laboratory functions
Note: These categories cover the specialty requirements that come with working in specific industries such as agriculture, pharmaceutical production, and forensic science. You'll likely notice that most of the content here isn't covered by ASTM E1578-18.
17. Production management
| ||||||||||||||||||||||||||||||||||||||
18. Statistical trending and control charts
| ||||||||
19. Agriculture and food data management
| ||||||||||||||||||
20. Environmental data management
Not relevant to manufacturing labs.
21. Forensic case and data management
Not relevant to manufacturing labs.
22. Clinical and public health data management
Not relevant to manufacturing labs.
23. Veterinary data management
Not relevant to manufacturing labs.
24. Scientific data management
| ||||||||||||||||||||||
25. Health information technology
Not relevant to manufacturing labs.
A5. Technology and performance improvements
Note: These categories cover what ASTM E1578-18 considers to be the extended functions that support core laboratory workflow, but in this case the dominant theme is additional technology that supports workflow, as well as requirements that improve overall laboratory performance.
26. Instrument data systems functions
| ||||||||||||
27. Systems integration
| ||||||||||||||||||||||||||||||||||||||||
28. Laboratory scheduling and capacity planning
| ||||||||||||
29. Lean laboratory and continuous improvement
| ||||||||||
30. Artificial intelligence and smart systems
| ||||||||||||||||||||||||||
A6. Security and integrity of systems and operations
Note: These categories cover what ASTM E1578-18 largely considers to be "platform and administration support functions." Notably, most of the requirements here have something to do with ensuring the security and integrity of not only the system and its functions but also the data that it houses and modifies.
31. Data integrity
| ||||||||||||||||||||
32. Configuration management
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
33. System validation and commission
| ||||||||||||||||
34. System administration
| ||||||||||||||||||||||||||||||||||
35. Cybersecurity
| ||||||||||||||||
36. Information privacy
| ||||||||||||||||
A7. Putting those requirements to practical use and caveats
The LIMSpec covered laboratory informatics requirements organized into five broad categories, which are heavily influenced by the functional requirements checklist and Figure 3 of ASTM E1578-18 Standard Guide for Laboratory Informatics. However, the requirements listed prior are all based on not just the ASTM E1578 standard but also a wide variety of other standards, regulations, guidance documents, and standardized procedures (hereon referred to as "sources"). That ultimately means a foundational reasoning is provided for each requirement, not necessarily a "just because I want it" reasoning. As foundational requirements, this LIMSpec should thus operate as an excellent starting point for building your own software requirements specification or for researching the best laboratory informatics solution for your laboratory.
Software developer considerations
What does that mean for you? How can you best use this document? If you're a software developer for the laboratory industry, many of the sources referenced in these requirements should already be familiar to you. However, some of them may not be, and you'll probably want to at least familiarize yourself with them. Additionally, if you're developing a generic laboratory information management system (LIMS) or some other informatics solution, not tailored to a particular industry, most everything in chapters two, three, five, and six should largely be applicable to what you're doing with your commercial off-the-shelf (COTS) software solution. Definitely review the requirements items listed there and make sure the most important ones are part of your own software requirements specification. If the software solution you're developing is tailored to a particular industry (e.g., clinical or public health, pharmaceutical development, or heavy metals testing), you'll also want to examine chapter four. If you don't see many requirements for your industry listed (see the "Caveats" section later), you'll probably have additional research to conduct to see what additional sources will affect how you develop the functional and, particularly, non-functional requirements.
Buyer considerations
If you're a potential buyer of a laboratory informatics solution, this LIMSpec is also useful to you. Perhaps you know a bit about your laboratory's workflow and a few of the regulations and standards that influence how that workflow is conducted, but you're not entirely informed. Reviewing the five broad categories of requirements may be necessary to help further inform you regarding what's vital in regards to what a laboratory informatics solution should be capable of. Additionally, you can then use these requirements as a base for your laboratory's own requirements list. Using the categories and their subdivisions, you can then add those requirements that are unique to your laboratory and industry that are not sufficiently covered by the LIMSpec requirements. As you review the various options available to you and narrow down your search, your own list of requirements can be used as both as a personal checklist and as a requirements list you hand over to the vendor you query.
Software vendor selection
That said, the requirements you hand off to the vendor should be discussed a bit more. Software vendor selection can at times be a tedious yet necessary process, one which requires careful planning and best practices. This topic has been written about by both software developers and end users alike, and their experiences should play a role in how you select a vendor. What follows is bullet-pointed advice as offered by some of those developers and end users.[10][11][12][13][14][15][16][17]
- Have a clear business case and build your business needs into your laboratory's requirements.
- Be mindful of how detailed you get with your own business-based requirements and what you initially hand off to a vendor. If you're too specific with too many requirements, you may have trouble finding a vendor that matches up. Start with the essentials that involve your laboratory's processes, regulations, integrations, reporting, service needs, etc. As this LIMSpec is foundation-based, you have a good starting point in that regard. You can always get more detailed with requirements as you narrow down vendors.
- As discussed briefly in the introduction, you'll need to prioritize your needs somewhere between "critical" and "nice to have." The LIMSpec's requirements are largely critical for most purposes and can be marked as such. The requirements you add will have to be prioritized more carefully.
- You'll also want to perform some informal third-party information gathering about the vendors. Are reviews of the vendors trustworthy? Have peers had any interactions and success with the vendor? Does the vendor have the ability to scale to meet your needs?
- Schedule demonstrations of programs that seem like strong initial candidates. Make sure there is a question and answer session afterwards, and perform a post-demo evaluation.
- A formal request for proposal (RFP) may or may not be necessary, depending on the level of information you acquire prior. However, formally requesting pricing and clarification of maintenance and additional service costs is useful. Just don't let price be the only thing that guides you.
- Consider some of the intangibles. Does the vendor genuinely seem interested in your business and its needs? Do they communicate well and promptly? Do they seem flexible and able to accommodate a few special case requirements?
- Be sure to consider future needs as you anticipate potential laboratory expansion.
- Don't be afraid to choose a consultant to help you with the vendor selection process.
Caveats
First, note that this LIMSpec is still an evolving entity. Standards change. Regulations change. Procedures also change with such standards and regulations. That means that as those foundational characteristics shift, this set of requirements will have to also evolve. As such, do your homework and don't take everything you see here as fixed law. If you're responsible for investigating and/or purchasing a laboratory informatics system, be sure you have at least some familiarity with the primary industry your laboratory serves, and by extension the regulations and standards that affect it.
Second, the number of industry-specific applications of laboratory informatics software continues to grow, and with it also the regulations and standards that affect those specialty laboratories. As such, some industry-specific requirements may have been missed for lack of or too expensive public-facing sources. As mentioned with the first caveat, this version of LIMSpec is evolving, and as industry experts and researchers are able to provide additional feedback on this document, it will surely grow with more relevant sources. In other words, don't consider this complete, particularly if you're in a specialized laboratory industry. You may have to add more items based on you industry knowledge and insights.
A8. LIMSpec in Microsoft Word format
Microsoft Excel is often used as a tool to document requirements specifications. However, one downside to Microsoft Excel is its inability to handle multiple hyperlinks in the same cell. If you've looked over the LIMSpec in Appendix 1, you've likely noticed there are multiple hyperlinks to regulations, specifications, and guidance documents in the first column of the tables. Translating these wiki-based documents to Excel makes for a challenge when trying to maintain those hyperlinks. As they add value to not only your laboratory's requirements research but also to vendors' understanding of the sources for your requirements, it was decided the hyperlinks should be maintained in any portable version. As such, a Microsoft Word version was created.
You can download a copy of the Microsoft Word version of LIMSpec from LIMSwiki by going to this file page, right-clicking the URL under the white box, and selecting "Save link as..." (Alternatively, you can just click the link, open the file, and then save it.) A compromise was made between keeping the hyperlinks in the first column readable and leaving enough room in the third column for a vendor to provide a response. This response space admittedly may be a limiting factor for vendors wanting to include screenshots. If this situation arises, you may encourage the vendor to select the entire first column and delete it, then widening the response column.
Note that this downloadable version of LIMSpec is released under the same licensing terms as this guide. Please see the first paragraph of the download for more details.
References
- ↑ "specification". Merriam-Webster. Merriam-Webster, Inc. https://www.merriam-webster.com/dictionary/specification. Retrieved 07 December 2022.
- ↑ Bieg, D.P. (August 2014). "Introduction" (PDF). Requirements Management: A Core Competency for Project and Program Success. Project Management Institute. p. 3. https://www.pmi.org/-/media/pmi/documents/public/pdf/learning/thought-leadership/pulse/requirements-management.pdf. Retrieved 07 December 2022.
- ↑ "ISO/IEC/IEEE 29148:2018". International Organization for Standardization. November 2018. https://www.iso.org/standard/72089.html. Retrieved 07 December 2022.
- ↑ Seibert, P. (28 July 2011). "How do you write software requirements? What are software requirements? What is a software requirement?". HubTechInsider. https://hubtechinsider.wordpress.com/2011/07/28/how-do-you-write-software-requirements-what-are-software-requirements-what-is-a-software-requirement/. Retrieved 07 December 2022.
- ↑ Memon, A. (Spring 2010). "Software Requirements: Descriptions and specifications of a system" (PDF). University of Maryland. https://www.cs.umd.edu/~atif/Teaching/Spring2010/Slides/3.pdf. Retrieved 07 December 2022.
- ↑ Aasem, M.; Ramzan, M.; Jaffar, A. (2010). "Analysis and optimization of software requirements prioritization techniques". Proceedings from the 2010 International Conference on Information and Emerging Technologies: 1–6. doi:10.1109/ICIET.2010.5625687.
- ↑ Hirsch, J. (22 November 2013). "10 Steps To Successful Requirements Gathering". Phase2 Technology, LLC. https://www.phase2technology.com/blog/successful-requirements-gathering. Retrieved 07 December 2022.
- ↑ Burris, E. (2007). "Requirements Specification". CS451R, University of Missouri–Kansas City. University of Missouri–Kansas City. Archived from the original on 24 July 2019. https://web.archive.org/web/20190724173601/http://sce2.umkc.edu/BIT/burrise/pl/requirements/. Retrieved 07 December 2022.
- ↑ Hofmann, H.F.; Lehner, F. (2001). "Requirements engineering as a success factor in software projects". IEEE Software 18 (4): 58–66. doi:10.1109/MS.2001.936219.
- ↑ Pearce, O. (21 June 2016). "Software Vendor Selection: How to Define Your Requirements". Montrium Blog. Montrium, Inc. https://blog.montrium.com/blog/software-vendor-selection-defining-your-requirements. Retrieved 07 December 2022.
- ↑ Pearce, O. (23 June 2016). "Software Vendor Selection: Finding the Right Vendor". Montrium Blog. Montrium, Inc. https://blog.montrium.com/blog/software-vendor-selection-finding-the-right-vendor. Retrieved 07 December 2022.
- ↑ Pearce, O. (28 June 2016). "Software Vendor Selection: The Pitfalls and Successes of Vendor Demos". Montrium Blog. Montrium, Inc. https://blog.montrium.com/blog/software-vendor-selection-conducting-demonstrations. Retrieved 07 December 2022.
- ↑ Pearce, O. (5 July 2016). "Software Vendor Selection: Requesting Proposals & Quotes". Montrium Blog. Montrium, Inc. https://blog.montrium.com/blog/software-vendor-selection-requesting-proposals-quotes. Retrieved 07 December 2022.
- ↑ Persaud, D. (4 February 2016). "Business Requirements Gathering for Enterprise Software Selection". SelectHub Blog. Abuyo, Inc. https://www.selecthub.com/miscellaneous/technology-selection/business-requirements-gathering-enterprise-software-selection/. Retrieved 07 December 2022.
- ↑ Lichtenberger, A. (23 July 2012). "Six Steps for a Successful Vendor Selection". ITIL.org. https://blog.itil.org/2012/07/six-steps-for-a-successful-vendor-selection/. Retrieved 07 December 2022.
- ↑ Poon, L. (29 May 2015). "Insider’s Guide to LIMS Selection". Genologics Blog. GenoLogics Life Sciences Software Inc. https://www.genologics.com/blog/insiders-guide-to-lims-selection/. Retrieved 20 September 2019. [dead link]
- ↑ "How to Select an ELN for Biology R&D". Benchling, Inc. https://benchling.com/static/docs/resources/eln-for-biology-rnd.pdf. Retrieved 07 December 2022.