Difference between revisions of "Steam distillation"
Shawndouglas (talk | contribs) m (1 revision imported) |
Shawndouglas (talk | contribs) m (Text replacement - "CannabisQAwiki" to "LIMSwiki") |
||
| Line 1: | Line 1: | ||
{{wikipedia::Steam distillation}} | {{wikipedia::Steam distillation}} | ||
==Notes== | ==Notes== | ||
This article is a direct transclusion of [https://en.wikipedia.org/wiki/Steam_distillation the Wikipedia article] and therefore may not meet the same editing standards as | This article is a direct transclusion of [https://en.wikipedia.org/wiki/Steam_distillation the Wikipedia article] and therefore may not meet the same editing standards as LIMSwiki. | ||
<!---Place all category tags here--> | <!---Place all category tags here--> | ||
[[Category:Articles transcluded from other wikis]] | [[Category:Articles transcluded from other wikis]] | ||
[[Category:Scientific techniques]] | [[Category:Scientific techniques]] | ||
Latest revision as of 22:26, 28 February 2024

Steam distillation is a separation process that consists of distilling water together with other volatile and non-volatile components. The steam from the boiling water carries the vapor of the volatiles to a condenser; both are cooled and return to the liquid or solid state, while the non-volatile residues remain behind in the boiling container.
If, as is usually the case, the volatiles are not miscible with water, they will spontaneously form a distinct phase after condensation, allowing them to be separated by decantation or with a separatory funnel.[1]
Steam distillation can be used when the boiling point of the substance to be extracted is higher than that of water, and the starting material cannot be heated to that temperature because of decomposition or other unwanted reactions. It may also be useful when the amount of the desired substance is small compared to that of the non-volatile residues. It is often used to separate volatile essential oils from plant material.[2] for example, to extract limonene (boiling point 176 °C) from orange peels.
Steam distillation once was a popular laboratory method for purification of organic compounds, but it has been replaced in many such uses by vacuum distillation and supercritical fluid extraction. It is however much simpler and economical than those alternatives, and remains important in certain industrial sectors.[3]
In the simplest form, water distillation or hydrodistillation, the water is mixed with the starting material in the boiling container. In direct steam distillation, the starting material is suspended above the water in the boiling flask, supported by a metal mesh or perforated screen. In dry steam distillation, the steam from a boiler is forced to flow through the starting material in a separate container. The latter variant allows the steam to be heated above the boiling point of water (thus becoming superheated steam), for more efficient extraction.[4]
History

Steam distillation is used in many of the recipes given in the Kitāb al-Taraffuq fī al-ʿiṭr ('Book of Gentleness on Perfume'), also known as the Kitāb Kīmiyāʾ al-ʿiṭr wa-l-taṣʿīdāt ('Book of the Chemistry of Perfume and Distillations'), attributed to the early Arabic philosopher al-Kindi (c. 801–873).[5] Steam distillation was also used by the Persian philosopher and physician Avicenna (980–1037) to produce essential oils by adding water to rose petals and distilling the mixture.[6] The process was also used by al-Dimashqi (1256–1327) to produce rose water on a large scale.[7]
Principle
Every substance has some vapor pressure even below its boiling point, so in theory it could be distilled at any temperature by collecting and condensing its vapors. However, ordinary distillation below the boiling point is not practical because a layer of vapor-rich air would form over the liquid, and evaporation would stop as soon as the partial pressure of the vapor in that layer reached the vapor pressure. The vapor would then flow to the condenser only by diffusion, which is an extremely slow process.
Simple distillation is generally done by boiling the starting material, because, once its vapor pressure exceeds atmospheric pressure, that still vapor-rich layer of air will be disrupted, and there will be a significant and steady flow of vapor from the boiling flask to the condenser.
In steam distillation, that positive flow is provided by steam from boiling water, rather than by the boiling of the substances of interest. The steam carries with it the vapors of the latter.
The substance of interest does not need to be miscible water or soluble in it. It suffices that it has significant vapor pressure at the steam's temperature.
If the water forms an azeotrope with the substances of interest, the boiling point of the mixture may be lower than the boiling point of water. For example, bromobenzene boils at 156 °C (at normal atmospheric pressure), but a mixture with water boils at 95 °C.[8] However, the formation of an azeotrope is not necessary for steam distillation to work.
Applications

Steam distillation is often employed in the isolation of essential oils, for use in perfumes, for example. In this method, steam is passed through the plant material containing the desired oils. Eucalyptus oil, camphor oil and orange oil are obtained by this method on an industrial scale.[2] Steam distillation is a means of purifying fatty acids, e.g. from tall oils.[9]
Steam distillation is sometimes used in the chemical laboratory. Illustrative is a classic preparation of bromobiphenyl where steam distillation is used to first remove the excess benzene and subsequently to purifiy the brominated product.[10] In one preparation of benzophenone, steam is employed to first recover unreacted carbon tetrachloride and subsequently to hydrolyze the intermediate benzophenone dichloride into benzophenone, which is in fact not steam distilled.[11] In one preparation of a purine, steam distillation is used to remove volatile benzaldehyde from nonvolatile product.[12]
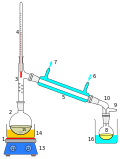
Equipment

On a lab scale, steam distillations are carried out using steam generated outside the system and piped through the mixture to be purified.[14][1] Steam can also be generated in-situ using a Clevenger-type apparatus.[15]
The Likens-Nickerson apparatus simultaneously performs steam distillation and extraction. It is typically used to isolate target organic compounds for further analysis.[16]
See also
- Azeotropic distillation
- Batch distillation
- Distillation
- Extractive distillation
- Fractional distillation
- Heteroazeotrope
- Herbal distillates
- Hydrodistillation
- Laboratory equipment
- Steam engine
- Steam stripping
- Supercritical fluid extraction
- Theoretical plate
References
- ^ a b H. T. Clarke, Anne W. Davis (1922). "Quinoline". Organic Syntheses. 2: 79. doi:10.15227/orgsyn.002.0079.
- ^ a b Fahlbusch, Karl-Georg; Hammerschmidt, Franz-Josef; Panten, Johannes; Pickenhagen, Wilhelm; Schatkowski, Dietmar; Bauer, Kurt; Garbe, Dorothea; Surburg, Horst (2003). "Flavors and Fragrances". Ullmann's Encyclopedia of Industrial Chemistry. doi:10.1002/14356007.a11_141. ISBN 3-527-30673-0.
- ^ Zeki Berk (2018): Food Process Engineering and Technology, 3rd edition. 742 pages. ISBN 978-0-12-812018-7 doi:10.1016/C2016-0-03186-8
- ^ Manuel G. Cerpa, Rafael B. Mato, María José Cocero, Roberta Ceriani, Antonio J. A. Meirelle, Juliana M. Prado, Patrícia F. Leal, Thais M. Takeuchi, and M. Angela A. Meireles (2008): "Steam distillation applied to the food industry". Chapter 2 of Extracting Bioactive Compounds for Food Products: Theory and Applications, pages 9–75. ISBN 9781420062397
- ^ Needham, Joseph (1980). Science and Civilisation in China. Volume 5: Chemistry and Chemical Technology. Part IV: Spagyrical Discovery and Invention: Apparatus, Theories and Gifts. Cambridge: Cambridge University Press. ISBN 9780521085731. p. 128, note h (cf. the reservations on the authenticity of the work in Needham 1980, p. 128, note d).
Translation of some of the recipes as quoted in the 14th-century cookbook Kanz al-fawāʾid fī tanwīʿ al-mawāʾid: Nasrallah, Nawal (2017). Treasure Trove of Benefits and Variety at the Table: A Fourteenth-Century Egyptian Cookbook. English Translation, with an Introduction and Glossary. Islamic History and Civilization. Vol. 148. Leiden: Brill. doi:10.1163/9789004349919. ISBN 978-90-04-34729-8. pp. 425–430. - ^ Shreve, Randolph Norris; Brink, Joseph Andrew (1977). Chemical Process Industries. McGraw-Hill. ISBN 978-0-07-057145-7.
Axe, Dr Josh; Rubin, Jordan; Bollinger, Ty (2020-06-01). The Chemistry of Essential Oils. Destiny Image Publishers. ISBN 978-0-7684-5702-5. - ^ Hill, Donald R. (1993). Islamic Science and Engineering. Edinburgh: Edinburgh University Press. ISBN 9781474469135. p. 85–87.
- ^ Martin's Physical Pharmacy & Pharmaceutical sciences, fifth edition, ISBN 0-7817-6426-2, Lippincott williams & wilkins
- ^ M.M. Chakrabarty (9 November 2003). Chemistry and Technology of Oils & Fats. Allied Publishers. pp. 12–. ISBN 978-81-7764-495-1.
- ^ M. Gomberg and W. E. Bachmann (1928). "p-Bromobiphenyl". Organic Syntheses. 8: 42. doi:10.15227/orgsyn.008.0042.
- ^ C. S. Marvel, W. M. Sperry (1928). "Benzophenone". Organic Syntheses. 8: 26. doi:10.15227/orgsyn.008.0026.
- ^ W. Klötzer, J. Stadlwieser, J. Raneburger (1986). "Electrophilic N-Amination of Imide Sodium Salts with O-Diphenylphosphinylhydroxylamine (DPH): 7-Aminotheophylline". Organic Syntheses. 64: 96. doi:10.15227/orgsyn.064.0096.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Sadgrove & Jones, A contemporary introduction to essential oils: Chemistry, bioactivity and prospects for Australian agriculture, Agriculture 5(1), 2015, doi:10.3390/agriculture5010048
- ^ Kenneth B. Wiberg (1960). Laboratory Technique in Organic Chemistry. McGraw-Hill. ISBN 0070700958.
{{cite book}}: ISBN / Date incompatibility (help) - ^ Walton & Brown, Chemicals From Plants, Imperial College Press, 1999.
- ^ Eikani, Mohammad H.; Golmohammad, Fereshteh; Rowshanzamir, Soosan; Mirza, Mehdi (2005). "Recovery of water-soluble constituents of rose oil using simultaneous distillation–extraction". Flavour and Fragrance Journal. 20 (6): 555–558. doi:10.1002/ffj.1482. ISSN 1099-1026.
Notes
This article is a direct transclusion of the Wikipedia article and therefore may not meet the same editing standards as LIMSwiki.