Difference between revisions of "Journal:Knowledge of internal quality control for laboratory tests among laboratory personnel working in a biochemistry department of a tertiary care center: A descriptive cross-sectional study"
Shawndouglas (talk | contribs) (Created stub. Saving and adding more.) |
Shawndouglas (talk | contribs) (Finished adding rest of content.) |
||
| (2 intermediate revisions by the same user not shown) | |||
| Line 20: | Line 20: | ||
}} | }} | ||
{{Ombox math}} | {{Ombox math}} | ||
==Abstract== | ==Abstract== | ||
'''Introduction''': The [[clinical laboratory]] holds a central position in patient care, and as such, ensuring accurate [[laboratory]] test results is a necessity. Internal [[quality control]] (QC) ensures day-to-day laboratory consistency. However, unless practiced, the success of laboratory [[quality management system]]s (QMSs) cannot be achieved. This depends on the efforts and commitment of laboratory personnel for its implementation. Hence, the aim of this study was to find out the knowledge of internal QC for laboratory tests among laboratory personnel working in the Department of Biochemistry, B.P. | '''Introduction''': The [[clinical laboratory]] holds a central position in patient care, and as such, ensuring accurate [[laboratory]] test results is a necessity. Internal [[quality control]] (QC) ensures day-to-day laboratory consistency. However, unless practiced, the success of laboratory [[quality management system]]s (QMSs) cannot be achieved. This depends on the efforts and commitment of laboratory personnel for its implementation. Hence, the aim of this study was to find out the knowledge of internal QC for laboratory tests among laboratory personnel working in the Department of Biochemistry, B.P. Koirala Institute of Health Sciences (BPKIHS), a tertiary care center. | ||
'''Methods''': This was a descriptive cross-sectional study conducted from July 1, 2022 to August 30, 2022 after receiving ethical approval from the Institutional Review Committee (Reference number: 2341/022). A semi-structured questionnaire was used to assess knowledge on internal QC. Three non-respondents were excluded. The operational definition of the knowledge domain was set before finalizing the questionnaire. The convenience sampling method was used. Point estimate and 95% confidence interval were calculated. | '''Methods''': This was a descriptive cross-sectional study conducted from July 1, 2022 to August 30, 2022 after receiving ethical approval from the Institutional Review Committee (Reference number: 2341/022). A semi-structured questionnaire was used to assess knowledge on internal QC. Three non-respondents were excluded. The operational definition of the knowledge domain was set before finalizing the questionnaire. The convenience sampling method was used. Point estimate and 95% confidence interval were calculated. | ||
| Line 38: | Line 32: | ||
==Introduction== | ==Introduction== | ||
The [[clinical laboratory]] plays a central role in providing [[information]] about the health of patients for appropriate prevention, diagnosis, and management of diseases.<ref name=":0">{{Cite book |last=Farr, J.M.; Shatkin, L. |year=2004 |title=Best Jobs for the 21st Century |publisher=JIST Works |page=490 |isbn=9781563709616}}</ref> For confidence in reports generated within the [[laboratory]], [[quality control]] (QC) plays a pivotal role. | |||
QC, which includes internal and external QC, describes a set of procedures used to check if laboratory results are reliable for the intended clinical use. Internal quality control (IQC) ensures day-to-day laboratory consistency.<ref>{{Cite journal |last=Mourtzikou |first=Antonia |last2=Stamouli |first2=Marilena |last3=Athanasiadi |first3=Elena |date=2013-04-01 |title=Improvement of Clinical Laboratory Services through Quality: |url=https://services.igi-global.com/resolvedoi/resolve.aspx?doi=10.4018/ijrqeh.2013040103 |journal=International Journal of Reliable and Quality E-Healthcare |language=ng |volume=2 |issue=2 |pages=38–46 |doi=10.4018/ijrqeh.2013040103 |issn=2160-9551}}</ref> Currently, the majority of laboratory results are generated by automated analyzers, with the shift in laboratory personnel’s duties from manual work to equipment maintenance, internal and external QC, instrument calibration, and [[Information management|data management]] of generated results.<ref>{{Cite book |last=Karkalousos, P.; Evangelopoulos, A. |year=2011 |editor-last=Ivanov, O. |title=Applications and Experiences of Quality Control |url=https://books.google.com.np/books?id=8eqcDwAAQBAJ |chapter=Chapter 17: Quality Control in Clinical Laboratories |publisher=InTech |pages=331–360 |isbn=9789533072364}}</ref> The success of implemented [[quality management system]]s (QMSs) in the laboratory cannot be achieved if not practiced properly, and this ultimately depends upon the knowledge, efforts, and commitment of laboratory personnel towards QMS implementation.<ref name=":1">{{Cite journal |last=Azhar, K.; Mumtaz, A.; Ibrahim, M. et al. |year=2012 |title=Knowledge, Attitude and Practice of Quality Assurance Among Medical Laboratory Technologists Working in Laboratories of Lahore |url=https://www.researchgate.net/publication/265185536_Knowledge_Attitude_and_Practice_of_Quality_Assurance_Among_Medical_Laboratory_Technologists_Working_in_Laboratories_of_Lahore |journal=Physicians Academy |volume=6 |issue=2 |pages=17–22}}</ref> | |||
The objective of this study was to find out the level of knowledge of IQC for laboratory tests among laboratory personnel working in the Department of Biochemistry, B.P. Koirala Institute of Health Sciences (BPKIHS), a tertiary care center. | |||
==Methods== | |||
A descriptive cross-sectional study was conducted in the Department of Biochemistry, BPKIHS, Dharan, Nepal after obtaining ethical approval from the Institutional Review Committee (Reference number: 2341/022). The data were collected from July 1, 2022 to August 30, 2022. Personnel working in laboratories under the Department of Biochemistry were included. The faculty in-charge, the technical staff in-charge, and those not willing to participate were excluded from the study. Convenience sampling was used. The sample size was calculated by using the following formula: | |||
<math>n = Z^{2} \times \frac{p \times q}{e^{2}}</math><br /> | |||
<math>n = 1.96^{2} \times \frac{0.50 \times 0.50}{0.1^{2}}</math><br /> | |||
<math>n = 97</math> | |||
Where n = minimum required sample size, Z = 1.96 at 95% confidence interval (CI), p = prevalence taken as 50% for maximum sample size calculation, q = 1-p, and e = margin of error 10%. Thus, the calculated sample size was 97. The above-calculated sample size was then adjusted for a finite population as: | |||
<math>n' = \frac{n}{1 + \frac{n -1}{N}}</math><br /> | |||
<math>n' = \frac{97}{1 + \frac{97 -1}{23}}</math><br /> | |||
<math>n' = 19</math> | |||
Where n' = adjusted sample size for a finite population, and N = the total number of laboratory personnel, 23. Thus, the final sample size was 19. However, a total of 20 sample size was used for this study. | |||
A semi-structured questionnaire was used for data collection. The questionnaire comprised two segments, including a socio-demographic profile and knowledge questions in regard to IQC. Socio-demographic variables of sex, education level, position held in the laboratory, and laboratory QC training ware taken. The operational definition of knowledge on IQC was set prior to finalizing the questionnaire, and the questionnaire was designed and finalized based on it. Questions on knowledge of IQC were based on the understanding of the purpose of IQC, the types of control materials, various control charts, how and when IQC should be performed, and interpretations of the Levey-Jennings Chart using the Westgard rule. There were a total of 20 questions to assess the IQC knowledge domain. The scores were calculated by assigning one point to each correct answer and zero to incorrect/don’t know/blank answers. A score of ≥10 was assigned as adequate knowledge, and ≤9 was assigned as inadequate knowledge.<ref name=":2">{{Cite web |last=Mekonnen, E. |date=2004 |title=Health Laboratory Management and Quality Assurance |url=https://www.cartercenter.org/resources/pdfs/health/ephti/library/lecture_notes/med_lab_tech_students/ln_hlth_lab_mgmnt_final.pdf |format=PDF |publisher=The Carter Center |accessdate=December 2022}}</ref> | |||
The participants were provided with information regarding the purpose of the study and what they was expected to do to minimize bias. The questionnaire was collected back within 24 hours. The confidentiality of each laboratory personnel filling out the questionnaire was maintained throughout the study. Data were entered and analyzed using Microsoft Excel 2011. Point estimate and 95% CI were calculated. | |||
==Results== | |||
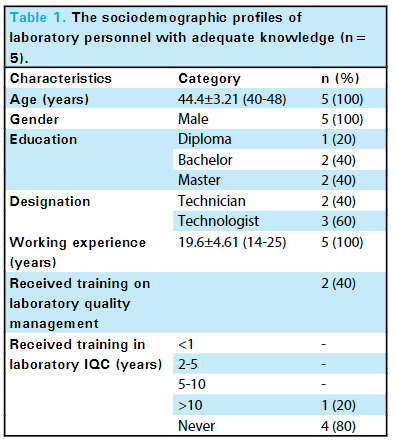
Among 20 laboratory personnel, only five (25%) (6.02-43.98, 95% confidence interval) had adequate knowledge of internal QC, with the mean knowledge score in this group as 12 ± 2.44 (Table 1). | |||
[[File:Fig1 Mishra JofNepMedAss23 61-258.png|395px]] | |||
{{clear}} | |||
{| | |||
| style="vertical-align:top;" | | |||
{| border="0" cellpadding="5" cellspacing="0" width="395px" | |||
|- | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |<blockquote>'''Table 1.''' The socio-demographic profiles of laboratory personnel with adequate knowledge (''n'' = 5).</blockquote> | |||
|- | |||
|} | |||
|} | |||
==Discussion== | |||
When we structure a questionnaire-based study, the main focus remains on a certain topic, with chosen questions unique to a particular setting and designed for a specific issue and topic.<ref name=":1" /> With the vital role that the laboratory holds in all health systems and in regard to the information about patient’s health, having confidence in the reports generated within the laboratory becomes crucial.<ref name=":0" /> IQC ensures day-to-day laboratory consistency and that the results from the laboratory tests are reliable; however, it is firstly a check of precision (i.e., reproducibility) but not necessarily accuracy.<ref name=":2" /> Well-developed and planned IQC program will continuously help ensures that the reports generated within the laboratory are accurate, reliable, and reproducible.<ref>{{Cite journal |last=McFarlane |first=A. |last2=Aslan |first2=B. |last3=Raby |first3=A. |last4=Moffat |first4=K. A. |last5=Selby |first5=R. |last6=Padmore |first6=R. |date=2015-12 |title=Internal Quality Control Practices in Coagulation Laboratories: recommendations based on a patterns‐of‐practice survey |url=https://onlinelibrary.wiley.com/doi/10.1111/ijlh.12397 |journal=International Journal of Laboratory Hematology |language=en |volume=37 |issue=6 |pages=729–738 |doi=10.1111/ijlh.12397 |issn=1751-5521}}</ref> However, if not practiced and adopted, the laboratory QMS fails. The practice of IQC majorly depends upon the laboratory personnel for its implementation, which ultimately depends upon their knowledge, attitude, and practiced behavior on IQC.<ref name=":1" /> According to the World Health Organization's (WHO's) Stepwise Laboratory Improvement Process Towards Accreditation (SLIPTA), performing IQC is one requirement that the laboratory should meet to achieve standards.<ref>{{Cite journal |last=Levey |first=Stanley |last2=Jennings |first2=E. R. |date=1950-11-01 |title=The use of Control Charts in the Clinical Laboratory* |url=https://academic.oup.com/ajcp/article/20/11_ts/1059/4828618 |journal=American Journal of Clinical Pathology |language=en |volume=20 |issue=11_ts |pages=1059–1066 |doi=10.1093/ajcp/20.11_ts.1059 |issn=0002-9173}}</ref> | |||
Our study findings suggested that the majority of the laboratory personnel had inadequate knowledge in regard to IQC. Very few (25%) laboratory personnel had adequate knowledge of IQC. This finding was in accordance with the work of Azhar ''et al.'', finding that the performance of day-to-day IQC practice is based on the knowledge and expertise obtained through job experience rather than sound knowledge.<ref name=":1" /> A similar explanation has also been found in the work of Dereje ''et al.''<ref name=":3">{{Cite journal |last=Dereje, M.G.; Yamirot, M.D.; Kumera, T.K. et al. |year=2018 |title=Assessment of Knowledge, Attitude and Practices of Medical Laboratory Professionals on the Use of Internal Quality Control for Laboratory Tests among Selected Health Centers in Addis Ababa Ethiopia |url=https://www.iomcworld.org/open-access/assessment-of-knowledge-attitude-and-practices-of-medical-laboratory-professionals-on-the-use-of-internal-quality-contro-47194.html |journal=Primary Health Care: Open Access |volume=8 |issue=2 |at=295}}</ref> The findings of Dargahi and Rezaiian also reported that laboratory application of their knowledge was obtained mostly by on-the-job experience.<ref>{{Cite journal |last=Dargahi, H.; Rezaiian, M. |year=2007 |title=Correlation between Knowledge, Attitude and Performance of the Employees with Quality Assurance System Implementation by the Employers |url=https://ijph.tums.ac.ir/index.php/ijph/article/view/2105 |journal=Iranian Journal of Public Health |volume=36 |issue=3 |pages=45–51}}</ref> A similar explanation could be implied in our study. Although not explored in our study, the inadequate content concerning laboratory quality management taught in bachelors of science medical laboratory technician (MLT) curriculum could be one of the reasons for inadequate knowledge.<ref name=":1" /> | |||
The mean age and working experience of the laboratory personnel in our study were higher than in other studies.<ref name=":1" /><ref name=":4">{{Cite journal |last=Ezenwaka |first=Chidum E |date=2000-06 |title=The quality of medical laboratory practice in Trinidad and Tobago, West Indies |url=https://onlinelibrary.wiley.com/doi/10.1046/j.1440-1762.2000.00369.x |journal=Journal of Quality in Clinical Practice |language=en |volume=20 |issue=2-3 |pages=75–78 |doi=10.1046/j.1440-1762.2000.00369.x |issn=1320-5455}}</ref> Despite their age and higher level of working experience, they were inadequately trained on IQC, with the majority of them never attaining any QC training after the completion of their formal education. This finding was similar to that of the work of Ezenwaka, where 59% of laboratory personnel admitting that they were inadequately trained.<ref name=":4" /> This might be due to an inadequate practice/policy of the institute and the laboratory to provide training to the laboratory personnel on a regular basis. Thus, strengthening the laboratory's IQC approach through proper and regular training and education of laboratory personnel seems a dire need in the particular context. We did not study the association of age and working experience on the average knowledge score; however, there are studies which show a positive association between these domains.<ref name=":3" /> A study by Mesfin ''et al.'' found that more than 33% of laboratory personnel fail to practice IQC.<ref>{{Cite journal |last=Mesfin |first=Eyob Abera |last2=Taye |first2=Binyam |last3=Belay |first3=Getachew |last4=Ashenafi |first4=Aytenew |last5=Girma |first5=Veronica |date=2017-10-10 |title=Factors Affecting Quality of Laboratory Services in Public and Private Health Facilities in Addis Ababa, Ethiopia |url=https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5655637/ |journal=EJIFCC |volume=28 |issue=3 |pages=205–223 |issn=1650-3414 |pmc=5655637 |pmid=29075171}}</ref> In order to sustain improvements in the quality of laboratories, a regular survey on medical laboratories should be conducted, which would question the accuracy and precision of laboratory analysis.<ref>{{Cite journal |last=Teka |first=Abaynesh |last2=Kibatu |first2=Girma |date=2012-3 |title=Quality of Liver and Kidney Function Tests among Public Medical Laboratories in Western Region of Amhara National Regional State of Ethiopia |url=https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3437976/ |journal=Ethiopian Journal of Health Sciences |volume=22 |issue=1 |pages=19–26 |issn=1029-1857 |pmc=3437976 |pmid=22984328}}</ref> | |||
Having regular IQC and total quality management training and meetings, conducting frequent and timely seminars/CMEs on the topic, and motivating a reward system within a laboratory to allow more participation during such training and educational sessions could be utilized as part of a strategy to enhance the knowledge amongst laboratory personnel. Numerous educational seminars, motivational sessions, and general improvement of IQC practices are needed to promote IQC use to reduce laboratory error at every step.<ref name=":3" /> | |||
The major factor for good IQC knowledge and practice is multifactorial. It involves education level, work experience, responsibility, and participation in various accreditation programs and the laboratory QMS itself.<ref name=":3" /> In order to achieve day-to-day reliability of the laboratory results, conducting a seminar or providing training opportunities on laboratory IQC and on total quality management could be effective strategies to help in enhancing the knowledge and overall practice towards IQC amongst the laboratory personnel. Conducting a multi-centric study involving laboratory personnel from different institutions would help us access the real knowledge status of laboratory personnel regarding laboratory IQC for better patient outcomes. | |||
==Conclusions== | |||
The prevalence of knowledge of IQC for laboratory tests among laboratory personnel working in the department of biochemistry was similar to other studies done in similar settings. Although the working experience is substantial, the laboratory personnel did not receive adequate training on laboratory IQC after the completion of their education. Hence, providing training opportunities on laboratory IQC can be reflected as a necessity in our current laboratory set-up. This could add value to the knowledge of IQC on laboratory personnel to ensure that the reports generated within the laboratory are accurate, reliable, and reproducible. | |||
==Acknowledgements== | |||
Authors would like to thank all the faculties, students, and laboratory staff of the Department of Biochemistry, BPKIHS. | |||
===Conflict of interest=== | |||
None stated. | |||
==References== | ==References== | ||
Latest revision as of 00:21, 21 February 2024
| Full article title | Knowledge of internal quality control for laboratory tests among laboratory personnel working in a biochemistry department of a tertiary care center: A descriptive cross-sectional study |
|---|---|
| Journal | Journal of Nepal Medical Association |
| Author(s) | Mishra, Bijaya; Lal Das, Binod K.; Khan, Seraj A.; Gelal, Basanta; Niraula, Apeksha; Chaudhari, Rejendra K.; Lamsal, Madhab |
| Author affiliation(s) | B.P. Koirala Institute of Health Sciences, Tribhuwan University |
| Primary contact | bjaya dot mp at gmail dot com |
| Year published | 2023 |
| Volume and issue | 61(258) |
| Page(s) | 167–70 |
| DOI | 10.31729/jnma.8040 |
| ISSN | 1815-672X |
| Distribution license | Creative Commons Attribution 4.0 International |
| Website | https://jnma.com.np/jnma/index.php/jnma/article/view/8040 |
| Download | https://jnma.com.np/jnma/index.php/jnma/article/download/8040/4607/38965 (PDF) |
|
|
This article contains rendered mathematical formulae. You may require the TeX All the Things plugin for Chrome or the Native MathML add-on and fonts for Firefox if they don't render properly for you. |
Abstract
Introduction: The clinical laboratory holds a central position in patient care, and as such, ensuring accurate laboratory test results is a necessity. Internal quality control (QC) ensures day-to-day laboratory consistency. However, unless practiced, the success of laboratory quality management systems (QMSs) cannot be achieved. This depends on the efforts and commitment of laboratory personnel for its implementation. Hence, the aim of this study was to find out the knowledge of internal QC for laboratory tests among laboratory personnel working in the Department of Biochemistry, B.P. Koirala Institute of Health Sciences (BPKIHS), a tertiary care center.
Methods: This was a descriptive cross-sectional study conducted from July 1, 2022 to August 30, 2022 after receiving ethical approval from the Institutional Review Committee (Reference number: 2341/022). A semi-structured questionnaire was used to assess knowledge on internal QC. Three non-respondents were excluded. The operational definition of the knowledge domain was set before finalizing the questionnaire. The convenience sampling method was used. Point estimate and 95% confidence interval were calculated.
Results: Among 20 laboratory personnel, five (25%) (6.02-43.98, 95% confidence interval) had adequate knowledge of internal QC. The mean knowledge score was 12 ± 2.44.
Conclusions: The prevalence of adequate knowledge of internal QC for laboratory tests among laboratory personnel working in the Department of Biochemistry at BPKIHS was similar to another study done in a similar setting.
Keywords: biochemistry, knowledge, laboratory personnel, quality control
Introduction
The clinical laboratory plays a central role in providing information about the health of patients for appropriate prevention, diagnosis, and management of diseases.[1] For confidence in reports generated within the laboratory, quality control (QC) plays a pivotal role.
QC, which includes internal and external QC, describes a set of procedures used to check if laboratory results are reliable for the intended clinical use. Internal quality control (IQC) ensures day-to-day laboratory consistency.[2] Currently, the majority of laboratory results are generated by automated analyzers, with the shift in laboratory personnel’s duties from manual work to equipment maintenance, internal and external QC, instrument calibration, and data management of generated results.[3] The success of implemented quality management systems (QMSs) in the laboratory cannot be achieved if not practiced properly, and this ultimately depends upon the knowledge, efforts, and commitment of laboratory personnel towards QMS implementation.[4]
The objective of this study was to find out the level of knowledge of IQC for laboratory tests among laboratory personnel working in the Department of Biochemistry, B.P. Koirala Institute of Health Sciences (BPKIHS), a tertiary care center.
Methods
A descriptive cross-sectional study was conducted in the Department of Biochemistry, BPKIHS, Dharan, Nepal after obtaining ethical approval from the Institutional Review Committee (Reference number: 2341/022). The data were collected from July 1, 2022 to August 30, 2022. Personnel working in laboratories under the Department of Biochemistry were included. The faculty in-charge, the technical staff in-charge, and those not willing to participate were excluded from the study. Convenience sampling was used. The sample size was calculated by using the following formula:
Where n = minimum required sample size, Z = 1.96 at 95% confidence interval (CI), p = prevalence taken as 50% for maximum sample size calculation, q = 1-p, and e = margin of error 10%. Thus, the calculated sample size was 97. The above-calculated sample size was then adjusted for a finite population as:
Where n' = adjusted sample size for a finite population, and N = the total number of laboratory personnel, 23. Thus, the final sample size was 19. However, a total of 20 sample size was used for this study.
A semi-structured questionnaire was used for data collection. The questionnaire comprised two segments, including a socio-demographic profile and knowledge questions in regard to IQC. Socio-demographic variables of sex, education level, position held in the laboratory, and laboratory QC training ware taken. The operational definition of knowledge on IQC was set prior to finalizing the questionnaire, and the questionnaire was designed and finalized based on it. Questions on knowledge of IQC were based on the understanding of the purpose of IQC, the types of control materials, various control charts, how and when IQC should be performed, and interpretations of the Levey-Jennings Chart using the Westgard rule. There were a total of 20 questions to assess the IQC knowledge domain. The scores were calculated by assigning one point to each correct answer and zero to incorrect/don’t know/blank answers. A score of ≥10 was assigned as adequate knowledge, and ≤9 was assigned as inadequate knowledge.[5]
The participants were provided with information regarding the purpose of the study and what they was expected to do to minimize bias. The questionnaire was collected back within 24 hours. The confidentiality of each laboratory personnel filling out the questionnaire was maintained throughout the study. Data were entered and analyzed using Microsoft Excel 2011. Point estimate and 95% CI were calculated.
Results
Among 20 laboratory personnel, only five (25%) (6.02-43.98, 95% confidence interval) had adequate knowledge of internal QC, with the mean knowledge score in this group as 12 ± 2.44 (Table 1).
|
Discussion
When we structure a questionnaire-based study, the main focus remains on a certain topic, with chosen questions unique to a particular setting and designed for a specific issue and topic.[4] With the vital role that the laboratory holds in all health systems and in regard to the information about patient’s health, having confidence in the reports generated within the laboratory becomes crucial.[1] IQC ensures day-to-day laboratory consistency and that the results from the laboratory tests are reliable; however, it is firstly a check of precision (i.e., reproducibility) but not necessarily accuracy.[5] Well-developed and planned IQC program will continuously help ensures that the reports generated within the laboratory are accurate, reliable, and reproducible.[6] However, if not practiced and adopted, the laboratory QMS fails. The practice of IQC majorly depends upon the laboratory personnel for its implementation, which ultimately depends upon their knowledge, attitude, and practiced behavior on IQC.[4] According to the World Health Organization's (WHO's) Stepwise Laboratory Improvement Process Towards Accreditation (SLIPTA), performing IQC is one requirement that the laboratory should meet to achieve standards.[7]
Our study findings suggested that the majority of the laboratory personnel had inadequate knowledge in regard to IQC. Very few (25%) laboratory personnel had adequate knowledge of IQC. This finding was in accordance with the work of Azhar et al., finding that the performance of day-to-day IQC practice is based on the knowledge and expertise obtained through job experience rather than sound knowledge.[4] A similar explanation has also been found in the work of Dereje et al.[8] The findings of Dargahi and Rezaiian also reported that laboratory application of their knowledge was obtained mostly by on-the-job experience.[9] A similar explanation could be implied in our study. Although not explored in our study, the inadequate content concerning laboratory quality management taught in bachelors of science medical laboratory technician (MLT) curriculum could be one of the reasons for inadequate knowledge.[4]
The mean age and working experience of the laboratory personnel in our study were higher than in other studies.[4][10] Despite their age and higher level of working experience, they were inadequately trained on IQC, with the majority of them never attaining any QC training after the completion of their formal education. This finding was similar to that of the work of Ezenwaka, where 59% of laboratory personnel admitting that they were inadequately trained.[10] This might be due to an inadequate practice/policy of the institute and the laboratory to provide training to the laboratory personnel on a regular basis. Thus, strengthening the laboratory's IQC approach through proper and regular training and education of laboratory personnel seems a dire need in the particular context. We did not study the association of age and working experience on the average knowledge score; however, there are studies which show a positive association between these domains.[8] A study by Mesfin et al. found that more than 33% of laboratory personnel fail to practice IQC.[11] In order to sustain improvements in the quality of laboratories, a regular survey on medical laboratories should be conducted, which would question the accuracy and precision of laboratory analysis.[12]
Having regular IQC and total quality management training and meetings, conducting frequent and timely seminars/CMEs on the topic, and motivating a reward system within a laboratory to allow more participation during such training and educational sessions could be utilized as part of a strategy to enhance the knowledge amongst laboratory personnel. Numerous educational seminars, motivational sessions, and general improvement of IQC practices are needed to promote IQC use to reduce laboratory error at every step.[8]
The major factor for good IQC knowledge and practice is multifactorial. It involves education level, work experience, responsibility, and participation in various accreditation programs and the laboratory QMS itself.[8] In order to achieve day-to-day reliability of the laboratory results, conducting a seminar or providing training opportunities on laboratory IQC and on total quality management could be effective strategies to help in enhancing the knowledge and overall practice towards IQC amongst the laboratory personnel. Conducting a multi-centric study involving laboratory personnel from different institutions would help us access the real knowledge status of laboratory personnel regarding laboratory IQC for better patient outcomes.
Conclusions
The prevalence of knowledge of IQC for laboratory tests among laboratory personnel working in the department of biochemistry was similar to other studies done in similar settings. Although the working experience is substantial, the laboratory personnel did not receive adequate training on laboratory IQC after the completion of their education. Hence, providing training opportunities on laboratory IQC can be reflected as a necessity in our current laboratory set-up. This could add value to the knowledge of IQC on laboratory personnel to ensure that the reports generated within the laboratory are accurate, reliable, and reproducible.
Acknowledgements
Authors would like to thank all the faculties, students, and laboratory staff of the Department of Biochemistry, BPKIHS.
Conflict of interest
None stated.
References
- ↑ 1.0 1.1 Farr, J.M.; Shatkin, L. (2004). Best Jobs for the 21st Century. JIST Works. p. 490. ISBN 9781563709616.
- ↑ Mourtzikou, Antonia; Stamouli, Marilena; Athanasiadi, Elena (1 April 2013). "Improvement of Clinical Laboratory Services through Quality:" (in ng). International Journal of Reliable and Quality E-Healthcare 2 (2): 38–46. doi:10.4018/ijrqeh.2013040103. ISSN 2160-9551. https://services.igi-global.com/resolvedoi/resolve.aspx?doi=10.4018/ijrqeh.2013040103.
- ↑ Karkalousos, P.; Evangelopoulos, A. (2011). "Chapter 17: Quality Control in Clinical Laboratories". In Ivanov, O.. Applications and Experiences of Quality Control. InTech. pp. 331–360. ISBN 9789533072364. https://books.google.com.np/books?id=8eqcDwAAQBAJ.
- ↑ 4.0 4.1 4.2 4.3 4.4 4.5 Azhar, K.; Mumtaz, A.; Ibrahim, M. et al. (2012). "Knowledge, Attitude and Practice of Quality Assurance Among Medical Laboratory Technologists Working in Laboratories of Lahore". Physicians Academy 6 (2): 17–22. https://www.researchgate.net/publication/265185536_Knowledge_Attitude_and_Practice_of_Quality_Assurance_Among_Medical_Laboratory_Technologists_Working_in_Laboratories_of_Lahore.
- ↑ 5.0 5.1 Mekonnen, E. (2004). "Health Laboratory Management and Quality Assurance" (PDF). The Carter Center. https://www.cartercenter.org/resources/pdfs/health/ephti/library/lecture_notes/med_lab_tech_students/ln_hlth_lab_mgmnt_final.pdf. Retrieved December 2022.
- ↑ McFarlane, A.; Aslan, B.; Raby, A.; Moffat, K. A.; Selby, R.; Padmore, R. (1 December 2015). "Internal Quality Control Practices in Coagulation Laboratories: recommendations based on a patterns‐of‐practice survey" (in en). International Journal of Laboratory Hematology 37 (6): 729–738. doi:10.1111/ijlh.12397. ISSN 1751-5521. https://onlinelibrary.wiley.com/doi/10.1111/ijlh.12397.
- ↑ Levey, Stanley; Jennings, E. R. (1 November 1950). "The use of Control Charts in the Clinical Laboratory*" (in en). American Journal of Clinical Pathology 20 (11_ts): 1059–1066. doi:10.1093/ajcp/20.11_ts.1059. ISSN 0002-9173. https://academic.oup.com/ajcp/article/20/11_ts/1059/4828618.
- ↑ 8.0 8.1 8.2 8.3 Dereje, M.G.; Yamirot, M.D.; Kumera, T.K. et al. (2018). "Assessment of Knowledge, Attitude and Practices of Medical Laboratory Professionals on the Use of Internal Quality Control for Laboratory Tests among Selected Health Centers in Addis Ababa Ethiopia". Primary Health Care: Open Access 8 (2): 295. https://www.iomcworld.org/open-access/assessment-of-knowledge-attitude-and-practices-of-medical-laboratory-professionals-on-the-use-of-internal-quality-contro-47194.html.
- ↑ Dargahi, H.; Rezaiian, M. (2007). "Correlation between Knowledge, Attitude and Performance of the Employees with Quality Assurance System Implementation by the Employers". Iranian Journal of Public Health 36 (3): 45–51. https://ijph.tums.ac.ir/index.php/ijph/article/view/2105.
- ↑ 10.0 10.1 Ezenwaka, Chidum E (1 June 2000). "The quality of medical laboratory practice in Trinidad and Tobago, West Indies" (in en). Journal of Quality in Clinical Practice 20 (2-3): 75–78. doi:10.1046/j.1440-1762.2000.00369.x. ISSN 1320-5455. https://onlinelibrary.wiley.com/doi/10.1046/j.1440-1762.2000.00369.x.
- ↑ Mesfin, Eyob Abera; Taye, Binyam; Belay, Getachew; Ashenafi, Aytenew; Girma, Veronica (10 October 2017). "Factors Affecting Quality of Laboratory Services in Public and Private Health Facilities in Addis Ababa, Ethiopia". EJIFCC 28 (3): 205–223. ISSN 1650-3414. PMC 5655637. PMID 29075171. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5655637/.
- ↑ Teka, Abaynesh; Kibatu, Girma (1 March 2012). "Quality of Liver and Kidney Function Tests among Public Medical Laboratories in Western Region of Amhara National Regional State of Ethiopia". Ethiopian Journal of Health Sciences 22 (1): 19–26. ISSN 1029-1857. PMC 3437976. PMID 22984328. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3437976/.
Notes
This presentation is faithful to the original, with changes to presentation, spelling, and grammar as needed. The PMCID and DOI were added when they were missing from the original reference.