Difference between revisions of "Main Page/Featured article of the week/2014"
Shawndouglas (talk | contribs) (Added last week's article of the week.) |
Shawndouglas (talk | contribs) (Ombox header) |
||
| (35 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
{{ombox | |||
| type = notice | |||
| text = If you're looking for other "Article of the Week" archives: 2014 - [[Main Page/Featured article of the week/2015|2015]] - [[Main Page/Featured article of the week/2016|2016]] - [[Main Page/Featured article of the week/2017|2017]] - [[Main Page/Featured article of the week/2018|2018]] - [[Main Page/Featured article of the week/2019|2019]] - [[Main Page/Featured article of the week/2020|2020]] - [[Main Page/Featured article of the week/2021|2021]] - [[Main Page/Featured article of the week/2022|2022]] - [[Main Page/Featured article of the week/2023|2023]] - [[Main Page/Featured article of the week/2024|2024]] | |||
}} | |||
==Featured article of the week archive - 2014== | ==Featured article of the week archive - 2014== | ||
| Line 12: | Line 17: | ||
<!-- Below this line begin pasting previous news --> | <!-- Below this line begin pasting previous news --> | ||
<h2 style="font-size:105%; font-weight:bold; text-align:left; color:#000; padding:0.2em 0.4em; width:50%;">Featured article of the week: June 30–July 6, 2014:</h2> | <h2 style="font-size:105%; font-weight:bold; text-align:left; color:#000; padding:0.2em 0.4em; width:50%;">Featured article of the week: December 22–28:</h2> | ||
<div style="padding:0.4em 1em 0.3em 1em;"> | |||
<div style="float: left; margin: 0.5em 0.9em 0.4em 0em;">[[File:Konelab60i.png|240px]]</div> | |||
'''[[Clinical chemistry]]''' (sometimes referred to as '''chemical pathology''') is the area of [[clinical pathology]] that is generally concerned with analysis of bodily fluids. The discipline originated in the late nineteenth century with the use of simple chemical tests for various components of blood and urine. Subsequent to this, other techniques were applied including the use and measurement of enzyme activities, spectrophotometry, [[electrophoresis]], and [[immunoassay]]. | |||
Today [[Clinical laboratory|clinical laboratories]] are now highly automated to accommodate the high workload typical of a hospital laboratory or [[reference laboratory]]. A large clinical laboratory will accept samples for up to about 700 different kinds of tests. Even the largest of laboratories rarely do all these tests themselves, and some must be referred to other labs. This large array of tests can be further sub-categorized into sub-specialties like general or routine chemistry, special chemistry, clinical endocrinology, [[toxicology]], therapeutic drug monitoring, urinalysis, and fecal analysis. ('''[[Clinical chemistry|Full article...]]''')<br /> | |||
</div> | |||
|- | |||
|<br /><h2 style="font-size:105%; font-weight:bold; text-align:left; color:#000; padding:0.2em 0.4em; width:50%;">Featured article of the week: December 15–21:</h2> | |||
<div style="padding:0.4em 1em 0.3em 1em;"> | |||
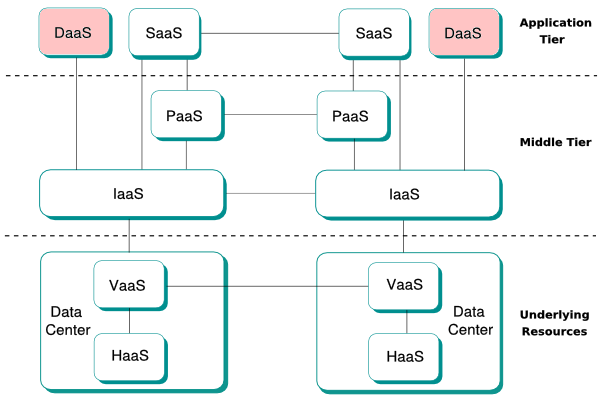
<div style="float: left; margin: 0.5em 0.9em 0.4em 0em;">[[File:Desktop-as-a-Service in Cloud Computing architectures.png|240px]]</div> | |||
'''[[Desktop virtualization]]''' is software technology that separates the desktop environment and associated application software from the physical client device that is used to access it. | |||
Desktop virtualization can be used in conjunction with application virtualization and user profile management systems (known as "user virtualization") to provide a comprehensive desktop environment management system. In this mode, all the components of the desktop are virtualized, which allows for a highly flexible and much more secure desktop delivery model. In addition, this approach supports a more complete desktop disaster recovery strategy as all components are essentially saved in the [[data center]] and backed up through traditional redundant maintenance systems. If a user's device or hardware is lost, the restore is much more straightforward and simple; all the components will be present at log-in from another device. Additionally, because no data is saved to the user's device, chances are low any critical data can be retrieved and compromised if the device is lost. ('''[[Desktop virtualization|Full article...]]''')<br /> | |||
</div> | |||
|- | |||
|<br /><h2 style="font-size:105%; font-weight:bold; text-align:left; color:#000; padding:0.2em 0.4em; width:50%;">Featured article of the week: December 08–14:</h2> | |||
<div style="padding:0.4em 1em 0.3em 1em;"> | |||
<div style="float: left; margin: 0.5em 0.9em 0.4em 0em;">[[File:US Navy 091001-N-6326B-090 Lyn A. Boulanger, a Naval Medical Center San Diego occupational therapist, assists Marine Corps Staff Sgt. Jesse A. Cottle, a bilateral amputee, while he practices swimming during endurance training.jpg|200px]]</div> | |||
A '''[[comprehensive outpatient rehabilitation facility]]''' ('''CORF''') is a non-residential facility established and operated solely to provide diagnostic, therapeutic, and restorative services to outpatients at a single, fixed location under the order and supervision of a physician. Loosely described as "follow-up medical rehabilitation" by the World Health Organization (WHO), the services offered by a "CORF" — a primarily U.S.-based descriptor — may also be found in "specialized rehabilitation wards or hospitals; rehabilitation centres; institutions such as residential mental and nursing homes, respite care centres, hospices, prisons, residential educational institutions, and military residential settings; or single or multiprofessional practices (office or clinic)" in the global health community. | |||
The U.S. [[Centers for Medicare and Medicaid Services]] (CMS) defines a CORF as "a nonresidential facility that is established and operated exclusively for the purpose of providing diagnostic, therapeutic, and restorative services to outpatients for the rehabilitation of injured, disabled, or sick persons, at a single fixed location, by or under the supervision of a physician." ('''[[Comprehensive outpatient rehabilitation facility|Full article...]]''')<br /> | |||
</div> | |||
|- | |||
|<br /><h2 style="font-size:105%; font-weight:bold; text-align:left; color:#000; padding:0.2em 0.4em; width:50%;">Featured article of the week: December 01–07:</h2> | |||
<div style="padding:0.4em 1em 0.3em 1em;"> | |||
<div style="float: left; margin: 0.5em 0.9em 0.4em 0em;">[[File:NIH Master Logo Vertical 2Color.png|140px]]</div> | |||
The '''[[National Institutes of Health]]''' ('''NIH''') is a biomedical research facility primarily located in Bethesda, Maryland, USA, operating as an agency of the [[United States Department of Health and Human Services]]. The NIH is the U.S. agency most responsible for biomedical and health-related research, primarily through its Intramural Research Program (IRP), which claims to be "the largest institution for biomedical science on earth." In addition to conducting its own research, the agency provides major biomedical research funding to non-NIH research facilities through its Extramural Research Program (ERP). For example, in 2003 the NIH and its extramural arm provided 28% of biomedical research funding spent annually in the U.S., or about $26.4 billion. | |||
The NIH comprises 27 separate institutes and centers that conduct research in different disciplines of biomedical science. The IRP is responsible for many scientific accomplishments, including the discovery of fluoride to prevent tooth decay, the use of lithium to manage bipolar disorder, and the creation of vaccines against hepatitis, ''Haemophilus influenzae'' (HIB), and human papillomavirus. The funding of NIH has at times been a source of contention in Congress, serving as a proxy for the political currents of the time. In fiscal year 2010, NIH spent $10.7 billion (not including temporary funding from the ARRA) on clinical research, $7.4 billion on genetics-related research, $6.0 billion on prevention research, $5.8 billion on cancer, and $5.7 billion on [[biotechnology]]. ('''[[National Institutes of Health|Full article...]]''')<br /> | |||
</div> | |||
|- | |||
|<br /><h2 style="font-size:105%; font-weight:bold; text-align:left; color:#000; padding:0.2em 0.4em; width:50%;">Featured article of the week: November 24–30:</h2> | |||
<div style="padding:0.4em 1em 0.3em 1em;"> | |||
<div style="float: left; margin: 0.5em 0.9em 0.4em 0em;">[[File:Nurse ict uganda.jpg|140px]]</div> | |||
A '''[[laboratory information system]] (LIS)''' is a software system that records, manages, and stores data for clinical [[laboratory|laboratories]]. A LIS has traditionally been most adept at sending laboratory test orders to lab instruments, tracking those orders, and then recording the results, typically to a searchable database. The standard LIS has supported the operations of public health institutions (like [[hospital|hospitals]] and clinics) and their associated labs by managing and reporting critical data concerning "the status of infection, immunology, and care and treatment status of patients." | |||
There is often confusion regarding the difference between a laboratory information system (LIS) and a [[laboratory information management system]] (LIMS). While the two laboratory informatics components are related, their purposes diverged early in their existences. Up until recently, LIMS and LIS have exhibited a few key differences, such as a LIS being designed primarily for processing and reporting data related to individual patients in a clinical setting, with a LIMS being traditionally designed to process and report data related to batches of samples from drug trials, water treatment facilities, and other entities that handle complex batches of data. However, distinctions between the two systems have faded somewhat as some LIMS vendors have adopted the case-centric information management normally reserved for a LIS, blurring the lines between the two components further. ('''[[Laboratory information system|Full article...]]''')<br /> | |||
</div> | |||
|- | |||
|<br /><h2 style="font-size:105%; font-weight:bold; text-align:left; color:#000; padding:0.2em 0.4em; width:50%;">Featured article of the week: November 17–23:</h2> | |||
<div style="padding:0.4em 1em 0.3em 1em;"> | |||
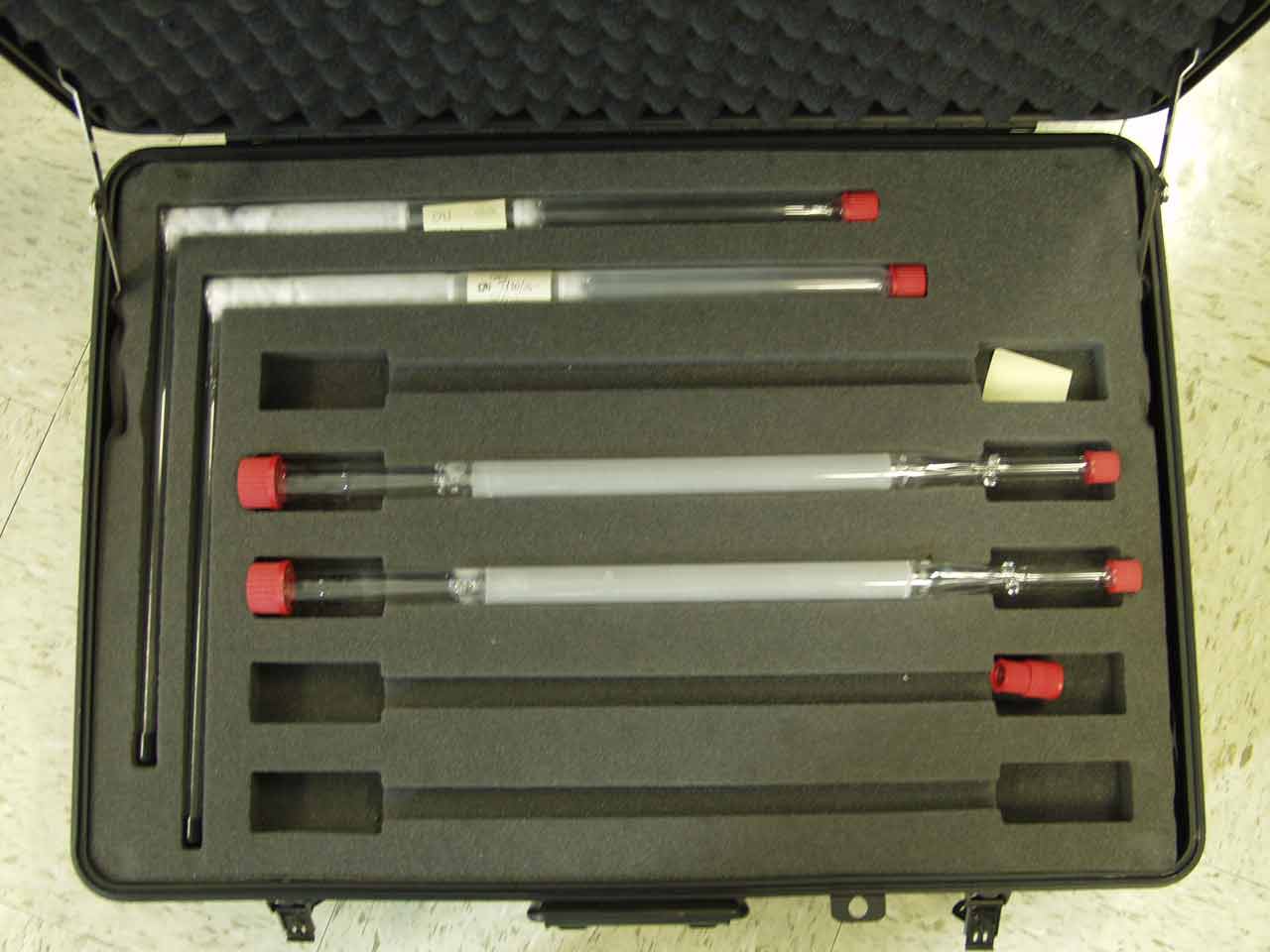
<div style="float: left; margin: 0.5em 0.9em 0.4em 0em;">[[File:Denuders.jpg|200px]]</div> | |||
A '''[[denuder]]''' is a cylindrical or annular conduit or tube internally coated with a reagent that selectively reacts with a stable flow of gas drawn through the conduit. The gas molecules diffuse to the walls while the [[analyte]] contained in the gas is transmitted outwards via laminar flow, collected, and analyzed. Effectiveness of the system depends primarily "on a complete discrimination between the gas species and particulate matter." | |||
Additional non-linear denuder geometries have also been tried with mixed results. Coiled configurations increased collection efficiency but lost larger particulate. A parallel multi-tube diffusion denuder has also been tried and found to increase collection efficiency. Other geometries include honeycombed, annular, and parallel plate. The development of the annular denuder in particular allowed researchers to overcome the inefficiencies of cylindrical denuders, allowing operation at larger flow rates (up to 30 times that of cylindrical denuders), shorter sampling periods, and less particle loss. ('''[[Denuder|Full article...]]''')<br /> | |||
</div> | |||
|- | |||
|<br /><h2 style="font-size:105%; font-weight:bold; text-align:left; color:#000; padding:0.2em 0.4em; width:50%;">Featured article of the week: November 10–16:</h2> | |||
<div style="padding:0.4em 1em 0.3em 1em;"> | |||
<div style="float: left; margin: 0.5em 0.9em 0.4em 0em;">[[File:Swine flu infection exponent by county June 2009.svg|200px]]</div> | |||
'''[[Infectious disease informatics]]''' ('''IDI''') is a multidisciplinary field of science that focuses on "the development of the science and technologies needed for collecting, sharing, reporting, analyzing, and visualizing infectious disease data and for providing data and decision-making support for infectious disease prevention, detection, and management." The field has expanded over time from analyzing [[public health laboratory]] data for potential disease vectors to a more robust syndromic surveillance of epidemiological factors and and to more advanced [[bioinformatics|bioinformatic]] approaches towards microbial, biomarker, and computational research. | |||
Infectious disease informatics can help tackle problems and tasks such as optimizing developed antimicrobials, improving vaccines, discovering biomarkers for transmissibility and clinical outcomes of infectious diseases, and developing research into host-pathogen interactions. A few unique considerations must be made in IDI informatics applications, including the confidentiality of any included personal health information (PHI) and the non-binary nature of user access privileges. For example, public health director of a certain region may be able to contribute a dataset for analysis, but they'll have to ensure the right balance of PHI to meet local, state, and federal regulations. ('''[[Infectious disease informatics|Full article...]]''')<br /> | |||
</div> | |||
|- | |||
|<br /><h2 style="font-size:105%; font-weight:bold; text-align:left; color:#000; padding:0.2em 0.4em; width:50%;">Featured article of the week: November 3–9:</h2> | |||
<div style="padding:0.4em 1em 0.3em 1em;"> | |||
<div style="float: left; margin: 0.5em 0.9em 0.4em 0em;">[[File:niosh.gif|200px]]</div> | |||
The '''[[National Institute for Occupational Safety and Health]]''' ('''NIOSH''') is the U.S. Federal agency responsible for conducting research and making recommendations for the prevention of work-related injury and illness. NIOSH is part of the [[Centers for Disease Control and Prevention]] (CDC) within the [[United States Department of Health and Human Services|U.S. Department of Health and Human Services]]. NIOSH provides national and world leadership to prevent work-related illness, injury, disability, and death by gathering information, conducting scientific research, and translating the knowledge gained into products and services. | |||
NIOSH is headquartered in Washington, D.C., with research laboratories and offices in Cincinnati, Ohio; Morgantown, West Virginia; Pittsburgh, Pennsylvania; Denver, Colorado; Anchorage, Alaska; Spokane, Washington; and Atlanta, Georgia. NIOSH is a professionally diverse organization with a staff ceiling of over 1,400 (as of 2005; operating with about 1,300 full-time employees) people representing a wide range of disciplines including epidemiology, medicine, industrial hygiene, safety, psychology, engineering, chemistry, and statistics. NIOSH was established to help ensure safe and healthful working conditions by providing research, information, education, and training in the field of occupational safety and health. ('''[[National Institute for Occupational Safety and Health|Full article...]]''')<br /> | |||
</div> | |||
|- | |||
|<br /><h2 style="font-size:105%; font-weight:bold; text-align:left; color:#000; padding:0.2em 0.4em; width:50%;">Featured article of the week: October 27–November 2:</h2> | |||
<div style="padding:0.4em 1em 0.3em 1em;"> | |||
<div style="float: left; margin: 0.5em 0.9em 0.4em 0em;">[[File:Martin Memorial Medical Center 001.JPG|200px]]</div> | |||
An '''[[ambulatory surgery center]]''' ('''ASC''') is a health care facility where surgical procedures not requiring [[Hospital|hospitalization]] are performed, with an expected duration of services less than 24 hours following admission. Such surgery is commonly less complicated than that requiring hospitalization. Avoiding hospitalization can result in cost savings to the party responsible for paying for the patient's health care. The ASC may also be known as an outpatient surgery center, same day surgery center, or surgicenter. | |||
An ASC specializes in providing surgical procedures, including certain pain management and diagnostic (e.g., colonoscopy) services in an outpatient setting. In simple terms, ASC-qualified procedures can be considered procedures that are more intensive than those done in the average doctor's office but not so intensive as to require a hospital stay. ('''[[Ambulatory surgery center|Full article...]]''')<br /> | |||
</div> | |||
|- | |||
|<br /><h2 style="font-size:105%; font-weight:bold; text-align:left; color:#000; padding:0.2em 0.4em; width:50%;">Featured article of the week: October 20–26:</h2> | |||
<div style="padding:0.4em 1em 0.3em 1em;"> | |||
<div style="float: left; margin: 0.5em 0.9em 0.4em 0em;">[[File:Datacenter-telecom.jpg|200px]]</div> | |||
A '''[[data center]]''' is a facility used to house computer systems and associated components such as telecommunications and storage systems. A data center generally includes redundant or backup power supplies, redundant data communications connections, environmental controls (e.g. air conditioning and fire suppression), and various security devices. Data centers vary in size, with some large data centers capable of using as much electricity as a "medium-size town." | |||
The main purpose of many data centers is for running applications that handle the core business and operational data of one or more organizations. Often those applications will be composed of multiple hosts, each running a single component. Common components of such applications include databases, file servers, application servers, and middleware. Such systems may be proprietary and developed internally by the organization or bought from enterprise software vendors. However, a data center may also solely be concerned with operations architecture or other services. ('''[[Data center|Full article...]]''')<br /> | |||
</div> | |||
|- | |||
|<br /><h2 style="font-size:105%; font-weight:bold; text-align:left; color:#000; padding:0.2em 0.4em; width:50%;">Featured article of the week: October 13–19:</h2> | |||
<div style="padding:0.4em 1em 0.3em 1em;"> | |||
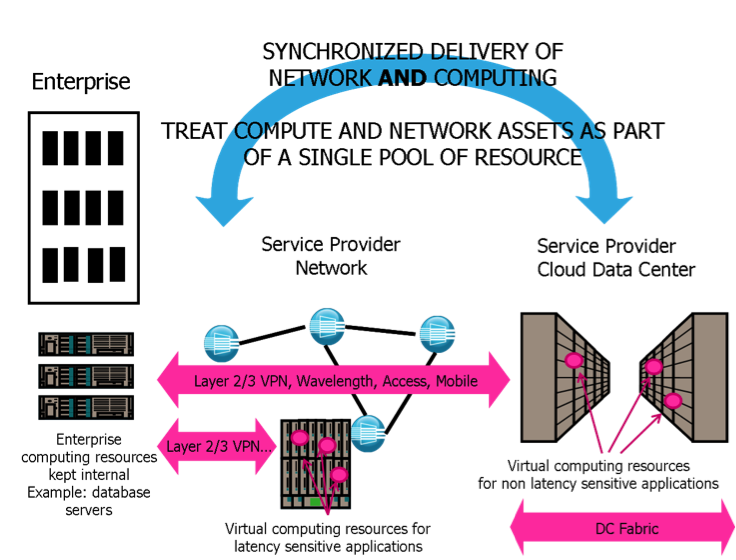
<div style="float: left; margin: 0.5em 0.9em 0.4em 0em;">[[File:CarrierCloud.png|180px]]</div> | |||
'''[[Carrier cloud]]''' is class of [[cloud computing]] service that merges the high-performance capabilities and reliability of a communications service provider's network with the lower costs and flexibility provided by traditional public cloud services. The carrier cloud attempts to remove the data bottleneck and security issues that often occur in and to the virtualized [[data center]] due to lack of control of data flow over the public Internet. | |||
Carrier cloud service is similar to public cloud service in that infrastructures are converged into a single, optimized computing package, and services are shared across a group or organization. Carrier cloud service, however, utilizes the existing and upgraded network structures of the communication service provider (CSP) to provide end-to-end services over their own network. Since the CSP more readily controls the data flow through its content delivery networks and/or dedicated virtual private networks, it can better manage issues with bandwidth, latency, and jitter. Additional "last mile" carrier-grade services already provided by CSPs in cities also "offset the latencies associated with cross-country or inter-continental backhaul." ('''[[Carrier cloud|Full article...]]''')<br /> | |||
</div> | |||
|- | |||
|<br /><h2 style="font-size:105%; font-weight:bold; text-align:left; color:#000; padding:0.2em 0.4em; width:50%;">Featured article of the week: October 6–12:</h2> | |||
<div style="padding:0.4em 1em 0.3em 1em;"> | |||
<div style="float: left; margin: 0.5em 0.9em 0.4em 0em;">[[File:Where the Trees Are - NASA Earth Observatory.jpg|180px]]</div> | |||
'''[[Forest informatics]]''' is a multidisciplinary field of science that "harnesses the power of computational and information technologies to organize and analyze biological data from research collections, experiments, remote sensing, modeling, database searches and instrumentation and deliver them to users throughout the world." Computational and information management technologies used to support decision-making activities in the field of forest informatics include decision support systems, mathematical modeling software, statistical and algorithmic analysis tools, geographic information systems, global positioning systems, and shared databases. | |||
Forestry informatics can help tackle problems and tasks such as optimizing harvest scheduling and crew assignment, computing wildfire risk indices, assessing forestry management guidelines, resolving log bucking problems, and developing and optimizing mathematical algorithms for ecological modeling. ('''[[Forest informatics|Full article...]]''')<br /> | |||
</div> | |||
|- | |||
|<br /><h2 style="font-size:105%; font-weight:bold; text-align:left; color:#000; padding:0.2em 0.4em; width:50%;">Featured article of the week: September 29–October 5:</h2> | |||
<div style="padding:0.4em 1em 0.3em 1em;"> | |||
<div style="float: left; margin: 0.5em 0.9em 0.4em 0em;">[[File:600px-International Electrotechnical Commission Logo.svg.png|160px]]</div> | |||
The '''[[International Electrotechnical Commission]]''' ('''IEC''') is a non-profit, non-governmental international standards organization that prepares and publishes international standards for many electrical devices, electronics, and other electrotechnology. IEC standards cover a vast range of technologies from power generation, transmission, and distribution to home appliances and office equipment, semiconductors, fibre optics, batteries, solar energy systems, marine energy systems, and nanotechnology. The IEC also manages three global conformity assessment systems that certify whether equipment, systems, or components conform to its international standards. IEC's membership comprises some 10,000 electrical and electronics experts from industry, government, academia, test labs, and others with an interest in the subject. | |||
The IEC charter embraces all electrotechnologies, including energy production and distribution systems, electronics, magnetic and electromagnetic devices, electroacoustic equipment, multimedia tools, telecommunication systems, and medical technology. The IEC also performs research and investigation into associated general disciplines such as terminology and symbols, electromagnetic compatibility (by its Advisory Committee on Electromagnetic Compatibility, ACEC), measurement and performance, research and development, safety, and the environmental sciences. ('''[[International Electrotechnical Commission|Full article...]]''')<br /> | |||
</div> | |||
|- | |||
|<br /><h2 style="font-size:105%; font-weight:bold; text-align:left; color:#000; padding:0.2em 0.4em; width:50%;">Featured article of the week: September 22–28:</h2> | |||
<div style="padding:0.4em 1em 0.3em 1em;"> | |||
<div style="float: left; margin: 0.5em 0.9em 0.4em 0em;">[[File:Johannes Cordua Arzt in seinem Studierzimmer.jpg|160px]]</div> | |||
A '''[[physician office laboratory]]''' ('''POL''') is a physician-, partnership-, or group-maintained [[laboratory]] that performs diagnostic tests or examines specimens in order to diagnose, prevent, and/or treat a disease or impairment in a patient as part of the physician practice. The POL shows up in primary care physician offices as well as the offices of specialists like urologists, hematologists, gynecologists, and endocrinologists. In many countries like the United States, the physician office laboratory is considered a [[clinical laboratory]] and is thus regulated by federal, state, and/or local laws affecting such laboratories. | |||
The workflow of a POL is similar to other clinical labs; the difference in workflows mostly comes down to the time spent in transporting the specimen to an outside lab and waiting for the processing. The in-office lab saves time in those parts of the process. Potential benefits of a POL include quicker access to test results for the clinician, greater efficiency of the clinical workflow, cheaper testing, and greater patient comfort and happiness. Potential disadvantages include the physician office being the only point-of-access, patients not feeling comfortable about the physician's office being the central repository of information, and the cost of meeting compliance requirements for local, state, and federal regulations. ('''[[Physician office laboratory|Full article...]]''')<br /> | |||
</div> | |||
|- | |||
|<br /><h2 style="font-size:105%; font-weight:bold; text-align:left; color:#000; padding:0.2em 0.4em; width:50%;">Featured article of the week: September 15–21:</h2> | |||
<div style="padding:0.4em 1em 0.3em 1em;"> | |||
<div style="float: left; margin: 0.5em 0.9em 0.4em 0em;">[[File:US-DeptOfHHS-Logo.svg|180px]]</div> | |||
The '''[[United States Department of Health and Human Services]]''' ('''HHS'''), also known as the '''Health Department''', is a cabinet-level department of the Federal government of the United States with the goal of protecting the health of all Americans and providing essential human services. Its motto is "Improving the health, safety, and well-being of America". Before the separate federal Department of Education was created in 1979, it was called the Department of Health, Education, and Welfare (HEW). | |||
HHS is administered by the Secretary of Health and Human Services, who is appointed by the President with the advice and consent of the Senate. The United States Public Health Service (PHS) is the main division of the HHS and is led by the Assistant Secretary for Health. The current Secretary, Kathleen Sebelius is the Vice-Chair of the United States Interagency Council on Homelessness. The Department of Health and Human Services is a member of the Council, which is dedicated to preventing and ending homelessness in America. | |||
As of May 2013, CMS administers 115 programs over 11 operating divisions, including the [[Centers for Disease Control and Prevention]] (CDC), the [[Centers for Medicare and Medicaid Services]] (CMS), and the [[National Institutes of Health]] (NIH). ('''[[United States Department of Health and Human Services|Full article...]]''')<br /> | |||
</div> | |||
|- | |||
|<br /><h2 style="font-size:105%; font-weight:bold; text-align:left; color:#000; padding:0.2em 0.4em; width:50%;">Featured article of the week: September 8–14:</h2> | |||
<div style="padding:0.4em 1em 0.3em 1em;"> | |||
<div style="float: left; margin: 0.5em 0.9em 0.4em 0em;">[[File:Keck Bioimaging Lab.jpg|240px]]</div> | |||
'''[[Bioimage informatics]]''' is a multidisciplinary sub-field of [[bioinformatics]] and computational biology that involves the development and use of computational techniques to analyze bioimages, especially cellular and molecular images, on a large scale fashion, with the goal of mining useful knowledge out of complicated and heterogeneous images and related metadata. | |||
The field of bioimage informatics is somewhat related to [[Imaging informatics|medical imaging informatics]], in so much as some of the advances in that field have found their way to the technology of analyzing bioimages. However, "it is very challenging to directly apply existing medical image analysis methods to ... bioimage informatics problems." Some of the challenges bioimages pose to researchers include the difficulty of analyzing at the cellular and molecular scales, the large size of the files, and the amount of time required to manually analyze the files. These challenges require automatic high-throughput analysis techniques, novel algorithms, and advanced systems to deal with the tasks of processing, storing, visualizing, and mining bioimages. ('''[[Bioimage informatics|Full article...]]''')<br /> | |||
</div> | |||
|- | |||
|<br /><h2 style="font-size:105%; font-weight:bold; text-align:left; color:#000; padding:0.2em 0.4em; width:50%;">Featured article of the week: September 1–7:</h2> | |||
<div style="padding:0.4em 1em 0.3em 1em;"> | |||
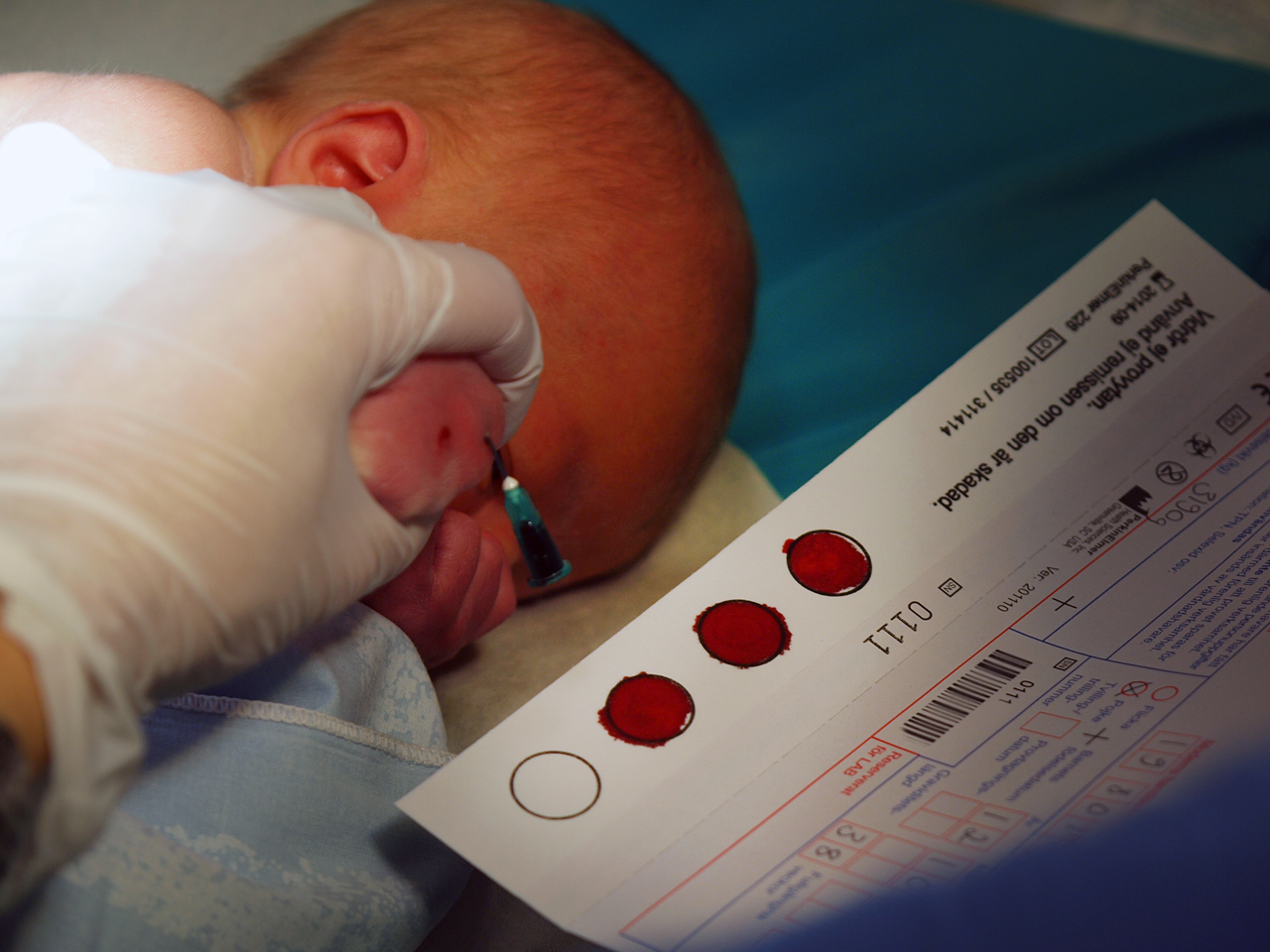
<div style="float: left; margin: 0.5em 0.9em 0.4em 0em;">[[File:PKU-test.jpg|240px]]</div> | |||
A '''[[biobank]]''' is a collection of biological samples (usually human) for use in research. The samples may also include personal medical and genealogical data. Sites for these collections come in different forms, typically based on the types of samples being stored and the scientific domain associated with them. These sites can be loosely categorized into two types: those based on biological specimens from patients and donors, and those specifically designed to aid in population-based research. | |||
Biobanks give researchers access to data representing larger numbers of people than could be analyzed in previously used systems. Furthermore, samples in biobanks and the data derived from those samples can often be used by multiple researchers for multiple purposes. Large collections of samples representing tens or hundreds of thousands of individuals are necessary to conduct certain studies. However, these activities come with their share of questions regarding research and medical ethics, and they have provoked discussions in some community circles. While viewpoints on what constitutes appropriate biobank ethics diverge, consensus has been reached that relying on biobanks without carefully considered governing principles and policies could negatively impact communities participating in biobank programs. ('''[[Biobank|Full article...]]''')<br /> | |||
</div> | |||
|- | |||
|<br /><h2 style="font-size:105%; font-weight:bold; text-align:left; color:#000; padding:0.2em 0.4em; width:50%;">Featured article of the week: August 25–31:</h2> | |||
<div style="padding:0.4em 1em 0.3em 1em;"> | |||
<div style="float: left; margin: 0.5em 0.9em 0.4em 0em;">[[File:Translational Research Center (TRC) of the Perelman School of Medicine.jpg|240px]]</div> | |||
'''[[Translational research]]''' is research that helps to make findings from basic science useful for practical applications that enhance human health and well-being. It is practiced in fields such as environmental and agricultural science, as well as the health, behavioral, and social sciences. For example, in medicine and nursing it is used to "translate" findings in basic research quickly into medical and nursing practice and meaningful health outcomes. | |||
The [[National Institutes of Health]] (NIH) defines translational research as such: | |||
"Translational research includes two areas of translation. One is the process of applying discoveries generated during research in the laboratory, and in preclinical studies, to the development of trials and studies in humans. The second area of translation concerns research aimed at enhancing the adoption of best practices in the community. Cost-effectiveness of prevention and treatment strategies is also an important part of translational science." | |||
Hence, translational research is seen as a key component to finding practical applications, especially within healthcare. ('''[[Translational research|Full article...]]''')<br /> | |||
</div> | |||
|- | |||
|<br /><h2 style="font-size:105%; font-weight:bold; text-align:left; color:#000; padding:0.2em 0.4em; width:50%;">Featured article of the week: August 18–24:</h2> | |||
<div style="padding:0.4em 1em 0.3em 1em;"> | |||
<div style="float: left; margin: 0.5em 0.9em 0.4em 0em;">[[File:Doctor's Office in New Orleans.jpg|140px]]</div> | |||
A '''[[rural health clinic]]''' ('''RHC''') is a special facility designation of the U.S. [[Centers for Medicare and Medicaid Services]] (CMS), defined as a clinic in a non-urbanized area designated by the Health Resources and Services Administration as being in a health professional shortage or medically underserved area. | |||
In September 1999, nearly 3,500 RHCs were operating across 45 states. By January 2013, that number rose to nearly 3,800. RHCs were established by the Rural Health Clinic Services Act of 1977, otherwise known as Public Law 95-210. The program was established to address an inadequate supply of physicians serving Medicare beneficiaries and Medicaid recipients in rural areas and to increase the utilization of non-physician practitioners. To qualify as an RHC the facility must be located in a non-urban area, as described by the United States Census Bureau, and must be defined as being in a medically underserved area by one of several possibilities. ('''[[Rural health clinic|Full article...]]''')<br /> | |||
</div> | |||
|- | |||
|<br /><h2 style="font-size:105%; font-weight:bold; text-align:left; color:#000; padding:0.2em 0.4em; width:50%;">Featured article of the week: August 11–17:</h2> | |||
<div style="padding:0.4em 1em 0.3em 1em;"> | |||
<div style="float: left; margin: 0.5em 0.9em 0.4em 0em;">[[File:PACS-RIS Services.png|200px]]</div> | |||
A [[picture archiving and communication system]] (PACS) is a digital imaging system composed of a set of components that allow for the digital acquisition, archiving, communication, retrieval, processing, distribution, and display of medical images. The PACS may consist of only a few components or be sufficiently complex to handle a hospital or healthcare enterprise environment. Regardless, it must be durable enough for daily use in a clinical environment, integrate to and from several [[Imaging informatics#Diagnostic imaging modalities|medical imaging modalities]], and have sufficient workstations for technicians utilizing those modalities to perform their work inside and outside the radiology department. PACS benefit healthcare providers by digitally managing medical images, eliminating the need to manually file, retrieve, or transport film jackets. This often saves processing time in both the diagnostics and reporting related to the imagery, especially when integrated with speech recognition technology. ('''[[Picture archiving and communication system|Full article...]]''')<br /> | |||
</div> | |||
|- | |||
|<br /><h2 style="font-size:105%; font-weight:bold; text-align:left; color:#000; padding:0.2em 0.4em; width:50%;">Featured article of the week: August 4–10:</h2> | |||
<div style="padding:0.4em 1em 0.3em 1em;"> | |||
<div style="float: left; margin: 0.5em 0.9em 0.4em 0em;">[[File:Krew Frakcjonowana.jpg|140px]]</div> | |||
'''[[Cytopathology]]''' is a branch of cytology and pathology that studies and diagnoses diseases on the cellular level. While cytopathology is closely related to [[histopathology]], the main difference is diagnostic information gained from cytopathology is acquired from disaggregated cell preparations rather than solid tissue samples. | |||
A common application of cytopathology is the Pap smear, used as a screening tool, to detect precancerous cervical lesions and prevent cervical cancer. Cytopathology is also commonly used to investigate thyroid lesions, diseases involving sterile body cavities (peritoneal, pleural, and cerebrospinal), and a wide range of other body sites. It is usually used to aid in the diagnosis of cancer, but also helps in the diagnosis of certain infectious diseases and other inflammatory conditions. Cytopathology is generally used on samples of free cells or tissue fragments, in contrast to [[histopathology]], which studies whole tissues. | |||
Rudolf Ludwig Karl Virchow is considered by many to be one of the fathers of cellular pathology, remembered most for his collection of lectures on the topic, published as ''Cellular Pathology'' in 1858. ('''[[Cytopathology|Full article...]]''')<br /> | |||
</div> | |||
|- | |||
|<br /><h2 style="font-size:105%; font-weight:bold; text-align:left; color:#000; padding:0.2em 0.4em; width:50%;">Featured article of the week: July 28–August 3, 2014:</h2> | |||
<div style="padding:0.4em 1em 0.3em 1em;"> | |||
<div style="float: left; margin: 0.5em 0.9em 0.4em 0em;">[[File:Peripheral blood smear - stained and unstained.jpg|120px]]</div> | |||
'''[[Clinical pathology]]''' (US, UK, Ireland, Commonwealth, Portugal, Brazil, Italy), '''laboratory medicine''' (Germany, Romania, Poland, Eastern Europe), '''clinical analysis''' (Spain), or '''clinical/medical biology''' (France, Belgium, Netherlands, Austria, North and West Africa) is a medical specialty concerned with the diagnosis of disease based on the [[laboratory]] analysis of bodily fluids, such as blood, urine, and tissues using the tools of chemistry, microbiology, hematology, and molecular pathology. Clinical pathologists work in close collaboration with clinical scientists (clinical biochemists, clinical microbiologists, etc.), medical technologists, [[hospital]] administrators, and referring physicians to ensure the accuracy and optimal utilization of laboratory testing. This specialty requires a medical residency and should not be confused with biomedical science, which is not necessarily related to medicine. | |||
Clinical pathology is one of two major divisions of pathology, the other being [[anatomical pathology]]. Often, pathologists practice both anatomical and clinical pathology, a combination sometimes known as general pathology. The distinction between clinical and anatomic pathology is increasingly blurred by the introduction of technologies that require new expertise and the need to provide patients and referring physicians with integrated diagnostic reports. ('''[[Clinical pathology|Full article...]]''')<br /> | |||
</div> | |||
|- | |||
|<br /><h2 style="font-size:105%; font-weight:bold; text-align:left; color:#000; padding:0.2em 0.4em; width:50%;">Featured article of the week: July 21–July 27, 2014:</h2> | |||
<div style="padding:0.4em 1em 0.3em 1em;"> | |||
<div style="float: left; margin: 0.5em 0.9em 0.4em 0em;">[[File:Lymph_node_with_metastatic_melanoma_-_by_Gabriel_Caponetti,_MD.jpg|200px]]</div> | |||
'''[[Anatomical pathology]]''' (or '''Anatomic pathology''') is a medical specialty that is concerned with the gross, microscopic, chemical, immunologic, and molecular examination of organs, tissues, and whole bodies (as in autopsy) to determine the presence of disease. Italian scientist Giovanni Battista Morgagni is widely considered the founding father of anatomic pathology. | |||
Anatomical pathology is one of two branches of pathology, the other being [[clinical pathology]], the diagnosis of disease through the laboratory analysis of bodily fluids. Often, pathologists practice both anatomical and clinical pathology, a combination known as general pathology. The distinction between anatomic and clinical pathology is increasingly blurred by the introduction of technologies that require new expertise and the need to provide patients and referring physicians with integrated diagnostic reports. | |||
The procedures used in anatomic pathology include gross examination, [[histopathology]], immunohistochemistry, ''in situ'' hybridization, [[cytopathology]], electron microscopy, tissue cytogenetics, and flow immunophenotyping. Anatomic pathology differs from clinical pathology in several ways, often led by the differentiation in [[laboratory]] workflow. ('''[[Anatomical pathology|Full article...]]''')<br /> | |||
</div> | |||
|- | |||
|<br /><h2 style="font-size:105%; font-weight:bold; text-align:left; color:#000; padding:0.2em 0.4em; width:50%;">Featured article of the week: July 14–July 20, 2014:</h2> | |||
<div style="padding:0.4em 1em 0.3em 1em;"> | |||
<div style="float: left; margin: 0.5em 0.9em 0.4em 0em;">[[File:Image 7 Information Relationship Model.jpg|200px]]</div> | |||
'''[[Information]]''', in its most restricted technical sense, is a sequence of symbols that can be interpreted as a message, recorded as signs, or transmitted as signals. Conceptually, information is the message (utterance or expression) being conveyed. Therefore, in a general sense, information is "knowledge communicated or received concerning a particular fact or circumstance." | |||
From the stance of information theory, information is taken as a sequence of symbols from an alphabet, say an input alphabet χ, and an output alphabet ϒ. Information processing consists of an input-output function that maps any input sequence from χ into an output sequence from ϒ. The mapping may be probabilistic or determinate. It may have memory or be memoryless. | |||
Information cannot be predicted and resolves uncertainty. The uncertainty of an event is measured by its probability of occurrence and is inversely proportional to that. The more uncertain an event, the more information is required to resolve uncertainty of that event. The amount of information is measured in bits. The concept that ''information is the message'' has different meanings in different contexts. Thus the concept of information becomes closely related to notions of constraint, communication, control, data, form, instruction, knowledge, meaning, understanding, stimulation, pattern, perception, representation, and entropy. ('''[[Information|Full article...]]''')<br /> | |||
</div> | |||
|- | |||
|<br /><h2 style="font-size:105%; font-weight:bold; text-align:left; color:#000; padding:0.2em 0.4em; width:50%;">Featured article of the week: July 7–July 13, 2014:</h2> | |||
<div style="padding:0.4em 1em 0.3em 1em;"> | |||
<div style="float: left; margin: 0.5em 0.9em 0.4em 0em;">[[File:Medical Laboratory Scientist US NIH.jpg|240px]]</div> | |||
A '''[[clinical laboratory]]''', (sometimes referred to as a '''medical laboratory''') is a laboratory where tests are done on clinical specimens in order to get information about the health of a patient as pertaining to the diagnosis, treatment, and prevention of disease. The [[Clinical Laboratory Improvement Amendments]] (CLIA) program defines a clinical (medical) laboratory as "a facility that performs testing on materials derived from the human body for the purpose of providing information for the diagnosis, prevention, or treatment of any disease or impairment of, or assessment of the health of, human beings." | |||
The clinical laboratory at one level, whether chemistry or pathology, operates like many other testing laboratories. However, there are a number of operational differences between the clinical laboratory and the many other laboratories. One of these differences is the need to have a specific unidirectional workflow. This is intended to both minimize the risk of biohazard contamination, and to establish assurance that samples cross contamination is minimized. Another difference involves the regulations governing the management of patient data. This creates a significant challenge not generally experienced by other types of laboratories. ('''[[Clinical laboratory|Full article...]]''')<br /> | |||
</div> | |||
|- | |||
|<br /><h2 style="font-size:105%; font-weight:bold; text-align:left; color:#000; padding:0.2em 0.4em; width:50%;">Featured article of the week: June 30–July 6, 2014:</h2> | |||
<div style="padding:0.4em 1em 0.3em 1em;"> | <div style="padding:0.4em 1em 0.3em 1em;"> | ||
<div style="float: left; margin: 0.5em 0.9em 0.4em 0em;">[[File:Tanzania hospital information mgt system.jpg|240px]]</div> | <div style="float: left; margin: 0.5em 0.9em 0.4em 0em;">[[File:Tanzania hospital information mgt system.jpg|240px]]</div> | ||
Latest revision as of 15:38, 2 January 2024
|
|
If you're looking for other "Article of the Week" archives: 2014 - 2015 - 2016 - 2017 - 2018 - 2019 - 2020 - 2021 - 2022 - 2023 - 2024 |
Featured article of the week archive - 2014
Welcome to the LIMSwiki 2014 archive for the Featured Article of the Week.
Featured article of the week: December 22–28:Clinical chemistry (sometimes referred to as chemical pathology) is the area of clinical pathology that is generally concerned with analysis of bodily fluids. The discipline originated in the late nineteenth century with the use of simple chemical tests for various components of blood and urine. Subsequent to this, other techniques were applied including the use and measurement of enzyme activities, spectrophotometry, electrophoresis, and immunoassay. Today clinical laboratories are now highly automated to accommodate the high workload typical of a hospital laboratory or reference laboratory. A large clinical laboratory will accept samples for up to about 700 different kinds of tests. Even the largest of laboratories rarely do all these tests themselves, and some must be referred to other labs. This large array of tests can be further sub-categorized into sub-specialties like general or routine chemistry, special chemistry, clinical endocrinology, toxicology, therapeutic drug monitoring, urinalysis, and fecal analysis. (Full article...)
|