Difference between revisions of "Journal:Quality control of cannabis inflorescence and oil products: Response factors for the cost-efficient determination of ten cannabinoids by HPLC"
Shawndouglas (talk | contribs) m (10 revisions imported) |
Shawndouglas (talk | contribs) (→Notes: Cats) |
||
| Line 786: | Line 786: | ||
<!--Place all category tags here--> | <!--Place all category tags here--> | ||
[[Category: | [[Category:LIMSwiki journal articles (added in 2022)]] | ||
[[Category: | [[Category:LIMSwiki journal articles (all)]] | ||
[[Category: | [[Category:LIMSwiki journal articles on cannabis cannabinoids]] | ||
[[Category: | [[Category:LIMSwiki journal articles on cannabis testing]] | ||
Latest revision as of 23:42, 26 December 2023
| Full article title | Quality control of cannabis inflorescence and oil products: Response factors for the cost-efficient determination of ten cannabinoids by HPLCn |
|---|---|
| Journal | Talanta Open |
| Author(s) | Hall, Damian R.; Sinclair, Justin S.; Bhuyan, Deep J.; Khoo, Cheang; Li, Chun G.; Sarris, Jerome; Low, Mitchell |
| Author affiliation(s) | NICM Health Research Institute, Wentworth Institute, Florey Institute of Neuroscience and Mental Health |
| Primary contact | Email: Mitchell dot Low at westernsydney dot edu dot au |
| Year published | 2022 |
| Volume and issue | 5 |
| Article # | 100112 |
| DOI | 10.1016/j.talo.2022.100112 |
| ISSN | 2666-8319 |
| Distribution license | Creative Commons Attribution 4.0 International |
| Website | https://www.sciencedirect.com/science/article/pii/S2666831922000315 |
| Download | https://www.sciencedirect.com/science/article/pii/S2666831922000315/pdfft (PDF) |
Abstract
The quality control (QC) of medicinal cannabis should include quantification of as many cannabinoids as practicable in a routine analytical laboratory, to accurately reflect the quality of the product. However, the cost and availability of some cannabinoid standards is an impediment to their routine use. This work seeks to overcome this obstacle by analyzing samples using relative retention times (RRT) and relative response factors (RRF), relative to cannabidiol (CBD) and cannabidiolic acid (CBDA) reference standards which are readily available. A high-performance liquid chromatography-photodiode array (HPLC-PDA) method was developed to quantify 10 cannabinoids—tetrahydrocannabinol (Δ9-THC), delta-8-Tetrahydrocannabinol (Δ8-THC), delta-9-Tetrahydrocannabinolic acid A (THCA-A), cannabinol (CBN), cannabidiol (CBD), cannabidiolic acid (CBDA), cannabichromene (CBC), cannabidivarin (CBDV), cannabigerol (CBG), and cannabigerolic acid (CBGA)—in dried cannabis inflorescence and cannabis oil. This method was validated according to International Council for Harmonisation (ICH) guidelines.
The proposed method has detection limits ranging from 20 to 78 µg/g, which provided sufficient sensitivity for the panel of cannabinoids. Non-cannabinoid surrogate matrices were used for spike recovery studies to determine method accuracy; analyte recoveries for the inflorescence and oil ranged from 90.1 to 109.3% (inflorescence mean, 100.9%; oil mean, 99.6%). The RRT and RRF values, determined independently by three analysts, were comparable, indicating the method is robust. The validity of analysis using RRT and RRF was further confirmed by testing six inflorescence samples, as it was found that concentrations above the order of magnitude of the limit of quantitation (LoQ) agreed satisfactorily (range, 95.0 to 111.9%; mean, 100.0%) with the concentrations obtained through the conventional approach of multipoint calibration using pure standards. The proposed method is therefore suitable for the rapid and simple determination of a panel of 10 cannabinoids without having to repeatedly purchase every expensive pure standard. Accordingly, analysts in the medicinal cannabis field may explore the use of RRF and RRT for their methods and instruments.
Keywords: liquid chromatography, cannabis, cannabinoid, response factor, relative retention time
Introduction
Despite an extensive history of use as a medicinal plant spanning ancient cultures[1][2][3], cannabis use is contentious in many jurisdictions, as it has been considered a social drug of abuse since the mid-1930s.[4][5] Over the last two decades, meaningful legal, sociocultural, and economic change has led to the establishment of medicinal cannabis research programs in several countries, which have validated the therapeutic use of cannabis for indications, including chronic neuropathic pain, certain intractable epilepsies, the vomiting and spasticity of multiple sclerosis, and chemotherapy-induced nausea.[6] Further to this, the use of medicinal cannabis has expanded into pediatric and vulnerable patient groups[7][8][9], and regulated markets for recreational use have developed in some jurisdictions. Accordingly, quality control (QC) across the supply chain is increasingly important to ensure that cannabis products are safe and have well-defined chemical and therapeutic profiles.
Critically, the complex relationship between chemical profiles and therapeutic activity requires further exploration. Presently, the activities of the three most abundant neutral cannabinoids—tetrahydrocannabinol (Δ9-THC), cannabidiol (CBD), and cannabigerol (CBG)—have been studied closely, exhibiting numerous properties, including analgesic, anticonvulsant, and anti-inflammatory characteristics.[6][10] However, the full potential of medicinal cannabis may not be realized without leveraging the full diversity of cannabinoids. Over 140 cannabinoids have been identified, many of which have their own inherent pharmacological properties.[11][12] This includes the acidic cannabinoids, which have significant anticonvulsant activities, contrary to the historical perspective that they were inert precursors which only acquired activity after decarboxylating into the neutral cannabinoids.[13] Furthermore, the complexity of cannabis increases geometrically under the "entourage effect," which postulates that cannabinoids interact to modulate their therapeutic effects.[14][15]
An experimental basis for the entourage effect is provided by murine studies, which have demonstrated that binary combinations with acidic cannabinoids increase bioavailability, potency, and efficacy of neutral cannabinoids in epilepsy models.[16][17][18] Even authors exclusively preoccupied with neutral cannabinoids have demonstrated synergistic binary combinations.[19][20][21] Clinical evidence is also mounting, with a recent meta-analysis on observational studies of epileptic patients concluding that crude cannabis extracts yielded a greater reduction in seizure frequency and had fewer side-effects than equivalent doses of purified CBD.[22] However, as most extracts were only characterized to the extent of standardizing the CBD dose, information about other cannabinoids was absent or based on inference. Consequently, the authors’ attribution of the differences between the extracts and purified CBD to the entourage effect was speculative. It was not possible to evaluate if the effects of the other cannabinoids added together, comparable to merely increasing the dose of CBD, or if they magnified the effect to surpass what CBD could achieve alone. Evidently, to progress beyond studies of binary combinations or poorly characterized extracts, routine analyses capable of quantifying panels of cannabinoids could help to better inform the design and interpretation of future studies that investigate the entourage effect. A clinical understanding of this effect might subsequently inform the extent to which cannabinoids are screened during cannabis product quality control.
Several published methods are available for the separation and quantification of cannabinoids, with a variety of limitations which constrain their routine use. For the analysis of neutral cannabinoids, gas chromatography (GC) is simple, sensitive, and provides acceptable resolution.[23] However, GC is not immediately suitable for acidic cannabinoids, as they are poorly volatilized and rapidly undergo thermal decarboxylation into neutral cannabinoids.[24] Fortunately, this limitation can be surmounted by trimethylsilyl derivatization of the labile acid group.[24][25] Alternatively, some analysts have adopted liquid chromatography (LC) for the separation of cannabinoids in medicinal cannabis. Following separation by LC, detection can be achieved by mass spectrometry (MS) or by photodiode array (PDA). The MS detector enables the peak identity confirmation from their fragmentation patterns and relative ratios[26], and it is sufficiently specific to recognize coeluting impurities in complex matrices.[24] However, the required technical expertise, operation, and maintenance costs prohibit the use of MS for the routine analysis of cannabinoids. The ultraviolet–visible spectroscopy (UV-Vis) PDA detectors are much cheaper, require less operator expertise, and are widely available. Since cannabinoids contain UV chromophores[27], they are amenable to PDA detection. Moreover, the UV spectra may assist with compound identity confirmation and the measurement of peak purity, which aids in quantification.
Whichever detector is used, the elevated cost and limited availability of certified analytical reference standards for some cannabinoids remain impediments to their analysis. The cost can exceed $200 AUD per mg, and newly identified pharmacological leads in cannabis are possibly more expensive with significantly longer shipping times. To surmount this, some analysts have performed stereoselective microscale syntheses to obtain cannabinoids in a timelier manner[28][29], but this is beyond the remit of a typical QC lab. If the analysis of such cannabinoids is to become routine, the cost for their quantification must be mitigated.
To this end, this study aimed to develop and validate a high-performance liquid chromatography-photodiode array (HPLC-PDA) method for the determination of 10 cannabinoids in medicinal cannabis inflorescence and oil and to explore the feasibility of using relative retention times (RRT) for peak identification and relative response factors (RRF) for their quantification. By this approach, an initial once-off purchase of all the standards was required to establish the RRT and RRF between the cannabinoids and the reference compounds. This included CBD as a reference for neutral cannabinoids, and cannabidiolic acid (CBDA) as a reference for acidic cannabinoids; they were chosen as they are cheaper and available in many jurisdictions. Subsequently, the method may be routinely used in QC laboratories for the quantification of a panel of 10 cannabinoids, requiring only sparing amounts of the reference compounds.
Materials and methods
Reagents and standards
LC-grade acetonitrile and methanol (purity >99.9%) were sourced from Honeywell (North Ryde, NSW, AU). Formic acid (>98%), ammonium formate (>98%), and dichloromethane (>99.8%) were sourced from Sigma-Aldrich (Castle Hill, NSW, AU). Ultra-pure water (resistivity >18.0 MΩ.cm) was obtained from a Milli-Q Direct 9 system (Sigma-Aldrich). A mixed 250 µg/mL standard of neutral cannabinoids—Δ9-THC, CBD, CBG, delta-8-Tetrahydrocannabinol (Δ8-THC), cannabinol (CBN), cannabichromene (CBC), and cannabidivarin (CBDV)—and acidic cannabinoids—CBDA, delta-9-Tetrahydrocannabinolic acid A (THCA-A), and cannabigerolic acid (CBGA)—in acetonitrile was produced by Cayman Chemical Company (Ann Arbor, MI, USA) and distributed by Sapphire Bioscience (Redfern, NSW, AU). Primary standards of single cannabinoids were also from the Cayman Chemical Company: CBN, THCA-A, and CBDA were obtained as 1000 µg/mL acetonitrile solutions; CBDV, CBC and tetrahydrocannabivarin (THCV) were obtained as 1000 µg/mL methanol solutions; and CBD, Δ9-THC, Δ8-THC, CBG, and CBGA were sourced as anhydrous solids. All standards were stored at −20 °C and allowed to come to room temperature in a desiccator before use.
An extraction solvent of acetonitrile:methanol (4:1 v/v) was prepared fresh daily, as required for the dilution of standards and the extraction of samples. For the conventional multipoint calibration curve, a 50 µg/mL dilution was prepared directly from the original 250 µg/mL mixed standard, and serial dilutions with a factor of two covered the concentration range of 1.6 to 25 µg/mL. For the quantification by RRF, a 25 µg/mL working standard of CBD and CBDA was prepared from their primary standards. This working standard concentration was chosen to be in the same order of magnitude as the sample concentration obtained when a 10 mg/g CBD oil is extracted according to the "sample preparation" subsection, below. All dilutions were prepared in the extraction solvent.
Sample preparation
Six air-dried and coarsely ground cannabis inflorescences, denoted as samples A-F, were provided by Little Green Pharma (Kings Park, WA, Australia). To ensure representative sampling of the biomass, the inflorescences were mechanically processed to pass through a 24-mesh sieve (710 µm openings). Residual water content (mean 5.5% w/w; CV <3%) was verified by drying triplicate sub-samples (∼ 800 mg) of the air-dried inflorescence over phosphorous pentoxide in a vacuum desiccator; drying was to a constant mass (<2 mg difference between days 7 and 8). For the analysis of cannabinoids, air-dried samples (400 mg) prepared in extraction solvent (25 mL) were sonicated in a Powersonic 420 ultrasonic bath (Thermoline Scientific; Sydney, NSW, Australia) on low power at room temperature for 30 minutes. Extracts were passed through 0.45 µm Nylon syringe filters into 2 mL HPLC autosampler vials. Where necessary, the extraction solvent was used to prepare 1:9 v/v dilutions of these extracts, so that cannabinoids with high concentrations (15–150 mg/g) could be analyzed in the same batch as those at low concentrations (0.1–15 mg/g). Both the undiluted and the diluted extract solutions were analyzed.
Medicinal cannabis oil (Cannimed; Saskatoon, Saskatchewan, Canada) was supplied by Health House International (Perth, WA, AU). The claimed composition was 10 mg/g total THC and 10 mg/g total CBD. These totals are corrected for the mass loss due to decarboxylation to report the concentrations in terms of neutral cannabinoid equivalents: CBD Total (mg/g) = CBD (mg/g) + 0.877 × CBDA (mg/g); and THC Total (mg/g) = Δ9-THC (mg/g) + 0.877 × THCA-A (mg/g). Oil samples were prepared for analysis by dissolving 50 µg in dichloromethane (1 mL), which made it miscible with the extraction solvent (total, 25 mL), and was subsequently sonicated on low power for 10 minutes. Extracts were passed through 0.45 µm Nylon syringe filters before analysis.
Instrumentation and analytical method
A Shimadzu Prominence-i LC-2030C 3D Plus HPLC-PDA (Rydalmere, NSW, AU)—comprising of a low-pressure quaternary solvent system, an auto sampler, and a PDA detector—was used. Shimadzu LabSolutions (v5.93A) was used for instrument control, data acquisition, and processing. A Phenomenex Luna C18(2) (150 × 4.6 mm × 5 µm) analytical column with a Security C18 (20 × 4.6 mm × 5 µm) guard column (Lane Cove West, NSW, AU) was employed to achieve reversed phase separation. The column was maintained at 40 °C, with a mobile phase flow rate of 2.5 mL/min. The injection volume was 10 µL, and all standards and samples were injected in duplicate. Gradient elution employing mobile phase A (Milli-Q water buffered with 20 mM ammonium formate and 0.1% formic acid), mobile phase B (acetonitrile) and mobile phase C (methanol buffered with 10 mM ammonium formate and 0.05% formic acid) was used. The gradient program is summarized in Table 1, which includes two minutes of column rinse with the organic phases and two minutes of re-equilibration at the starting condition. During each run, the PDA was set to acquire data from 190 to 800 nm and the chromatograms was visualized at 232 nm.
| ||||||||||||||||||||||||||||||||||||
System suitability
System suitability criteria were established to routinely ensure that the chromatographic system functioned as specified for each batch. To this end, a minimum of six injections of the CBD and CBDA 25 µg/mL working standard was made throughout each batch. To pass, the coefficient of variation (CV) of the standard retention times and peak areas of the six injections must be <2%.
Analysis by relative retention times and relative response factors
Standard values for the RRT and RRF were determined from three independent analysts who each performed six replicate injections of the mixed cannabinoid at the working standard concentration. The reference standards were CBD for neutral cannabinoids and CBDA for acidic cannabinoids. Adjusted retention time (tR' ) is the difference between the analyte retention time (tR) and the void time (tvoid); tR' = tR - tvoid. The RRT of a generic cannabinoid denoted as "a," relative to the reference, is the ratio of their adjusted retention times; tR' (a) / tR' (reference). Likewise, the response factor (RF) is the cannabinoid peak area (A) divided by its concentration (C); RF = A / C. The RRF of a generic cannabinoid denoted as "a," relative to the reference, is the ratio of their response factors; RF(a) / RF(reference).
Quantitative analyses using RRT and RRF values required that the CBD and CBDA working standards be tested in every batch of analysis. Using the reference retention times and the known RRT values, the expected retention time for cannabinoid "a" is determined; tR (a, expected) = [tR (reference, standard) - t0] x RRT (a) + t0. UV-vis spectra can verify this peak identification. Subsequently, the unknown concentration of cannabinoid "a" can be calculated; C(a) = [A (a, sample) / A (reference, standard)] x [1 / RRF (a)]. This represents the x C (reference, standard) concentration in the extract solution which, using the precise mass extracted in the known volumetric flask, is converted into the concentration of the original sample; reported as the mass of the cannabinoid (mg or µg) relative to the mass of the air-dried inflorescence or oil sample (/g).
Method validation
Analytical method validation was informed by the International Council for Harmonisation (ICH).[30] For the dried inflorescence sample, validation was performed for the quantification of all 10 cannabinoids. However, for the oil matrix, validation was only performed for the cannabinoids present in the preparation, namely Δ9-THC, THCA-A, CBD, and CBDA.
Linearity of the detector response was evaluated from calibration curves, considering both the R2 and the significance of the intercept. The detection and quantification limits were determined as limit of detection (LoD) = 3.3 s / m and LoQ = 10 s / m, respectively; where s is the sample standard deviation of the lowest linear concentration (3.1 µg/mL), and m is the calibration slope. The instrument and method precision were determined as the CV from six replicate standard injections and sample preparations, respectively. Intermediate precision was evaluated from the pooled CV between three analysts who independently prepared the same samples six times on separate days. The accuracy of the method was determined by a recovery study (see the "Accuracy protocol" sub-section, below). The stability of the standard and sample extracts was tested for up to 24 hours and 48 hours, respectively. Finally, the quantitative results obtained using the multipoint calibration curve and RRF method were compared.
Accuracy protocol
Recoveries of the 10 cannabinoids were tested using chamomile as a surrogate matrix for the cannabis inflorescence. For the cannabis oil, the recoveries of Δ9-THC, THCA-A, CBD, and CBDA were tested using olive oil as a surrogate matrix. These surrogate matrices were extracted according to the "Sample preparation" sub-section (above) to confirm that they did not give rise to peaks at the retention times of interest. Triplicate preparations of the surrogate matrices were spiked with the individual cannabinoid standards at levels relative to a representative sample for each matrix: Δ9-THC, CBD, and CBGA were spiked at 50%, 100%, and 200% levels; THCA-A, CBDA, and CBG were spiked at the 100% level due to limited supply; and all other cannabinoids were spiked at their LoQ.
Results and discussion
Method development
To optimize sample preparation, a variety of extraction solvents were tested with duplicate extractions. The solvents trailed were methanol, ethanol, acetonitrile, ethyl acetate, methanol:water (1:1 v/v), and acetonitrile:methanol (4:1 v/v). Cannabinoid peak areas were maximized by ethyl acetate and acetonitrile:methanol. However, due to markedly mismatching the initial mobile phase condition, ethyl acetate gave rise to significant band broadening. Thus, acetonitrile:methanol (4:1 v/v) was selected as the extraction solvent, which is consistent with other extraction optimization reports.[25][31]
The CV contribution of the method preparation procedure to the total uncertainty was determined by performing six replicate extractions and analysis of a single cannabis inflorescence sample. Grinding the inflorescence to pass through a < 710 µm sieve before subsampling achieved a CV range of 1.2 to 3.6%. When subsampling without grinding, the CV unacceptably ranged from 7.6 to 23.6%, thus indicating the importance of preparing a homogeneous sample.
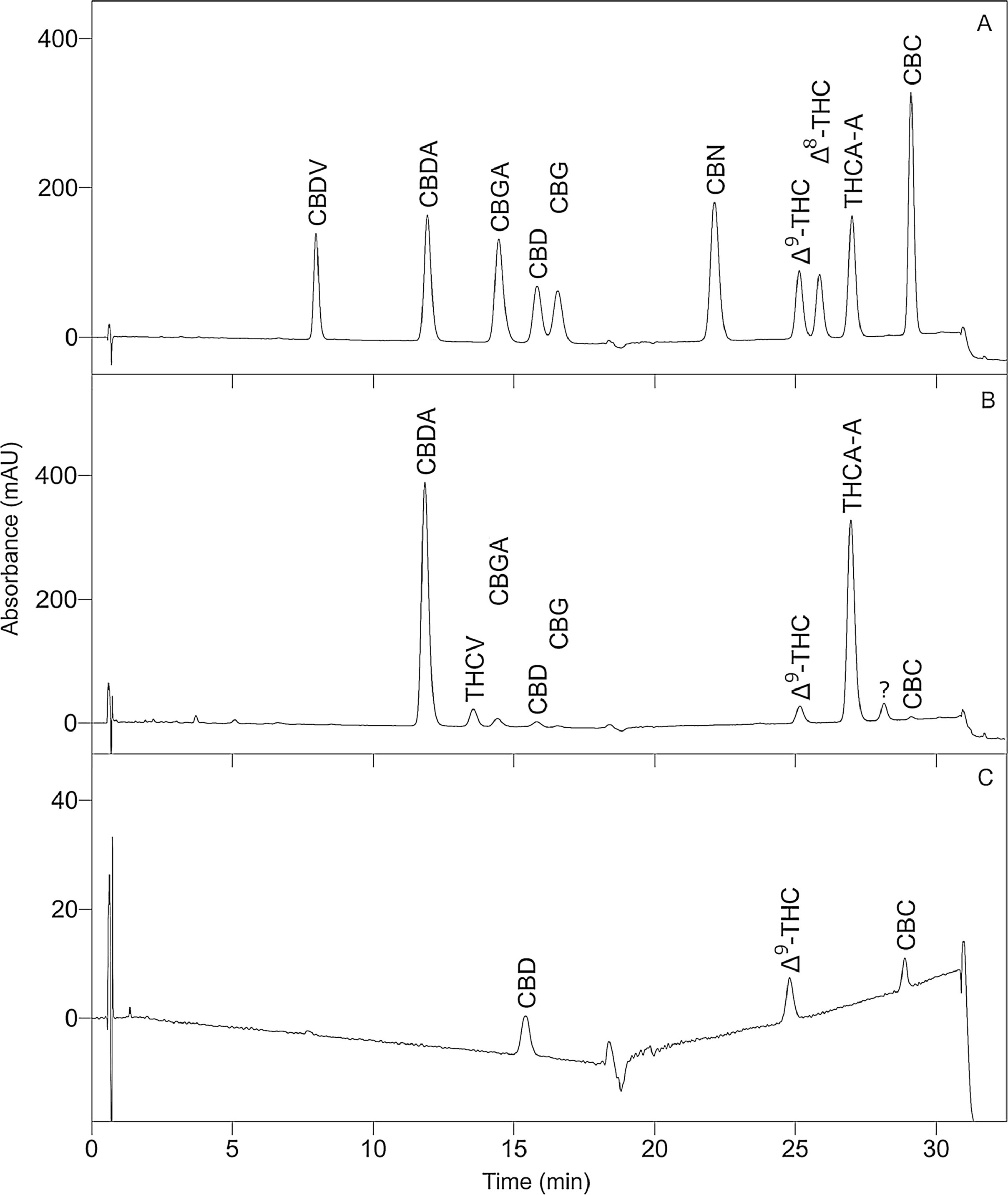
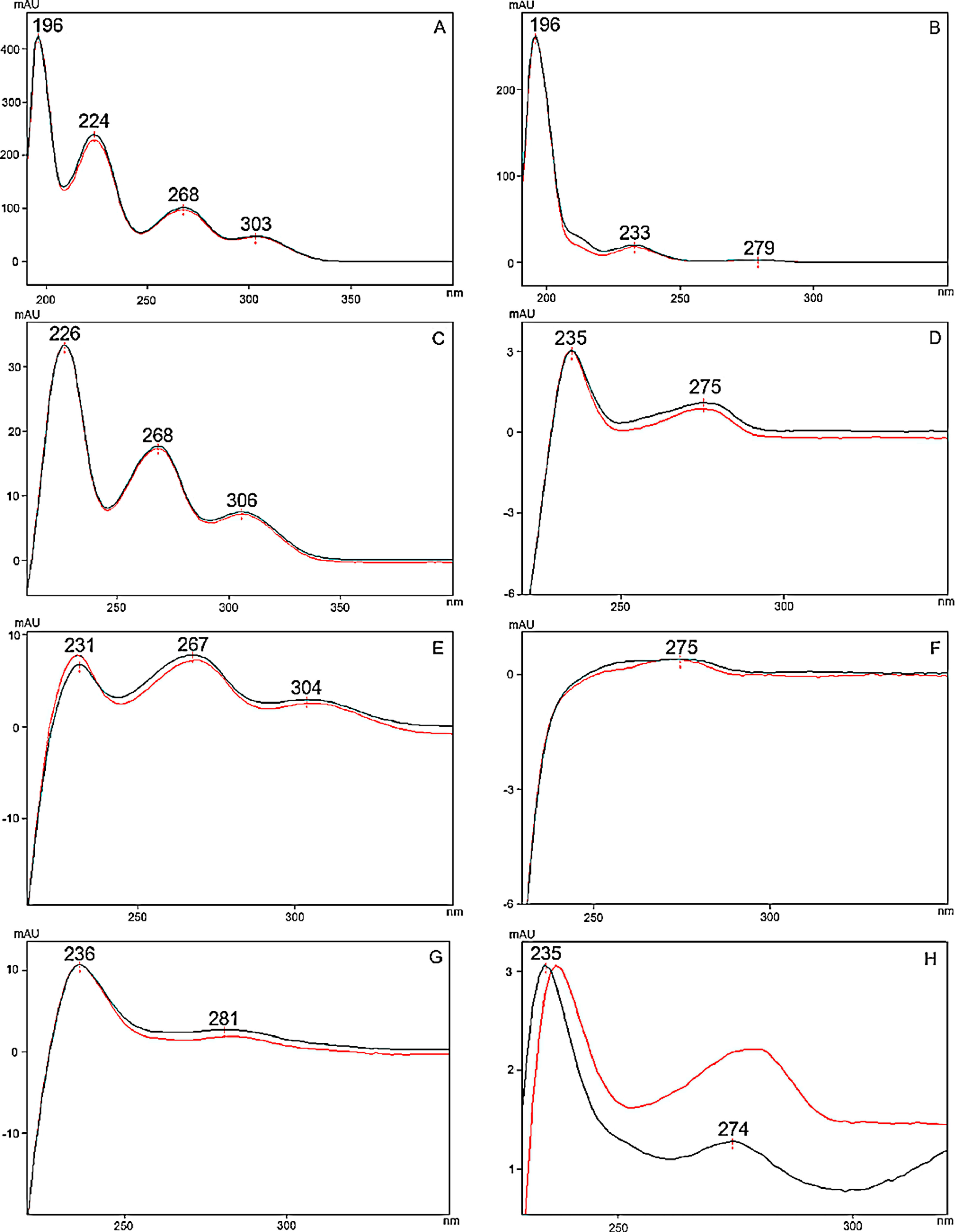
To optimize the chromatographic conditions, the method was iteratively developed. Baseline separation was achieved for eight of the 10 cannabinoid standards (resolution > 2.0); however, the CBD and CBG standard peaks overlapped slightly (resolution > 1.5, in all batches), as shown in Fig. 1A. Likewise, acceptable separation of cannabinoids in the extracts of cannabis inflorescence and cannabis oil were demonstrated in Fig. 1B and C, respectively. Whilst most matrix components eluted before the cannabinoids, a compound in inflorescence samples was observed to elute between CBDA and CBGA. This peak was identified to be tetrahydrocannabivarin (THCV, tR 13.70 minutes) by comparison with the UV spectrum and retention time obtained for the THCV standard. THCV was not included in the present method validation study as it was not part of the original selected set of analytes. When the analytes were sufficiently abundant in the sample, the UV spectra of their peaks were compared to that of the standard. As shown in Fig. 2, spectra superimposed closely, indicating good peak purity.
|
|
To optimize PDA detection, wavelengths corresponding to the λmax of the different cannabinoids, specifically 210, 232, and 270 nm, were considered. Whilst 210 nm has been used in other studies[24][25][32], it produced a sloping baseline in the present study due to the use of methanol (UV cut-off 210 nm) rather than exclusively using acetonitrile (UV cut-off 190 nm) as the organic component of the mobile phase. Instead, it was found that visualizing the chromatogram at 232 nm gave the best compromise between sensitivity and baseline noise. Some studies used 270 nm to improve sensitivity for the acidic cannabinoids[33], but this higher sensitivity is not required due to their relatively high abundance in the inflorescence samples. This high abundance was anticipated as acidic cannabinoids are the secondary metabolites synthesized in cannabis, whereas the neutral forms are produced by spontaneous decarboxylation.[34]
Linearity and calibration range
Considering the seven mixed-standard dilutions prepared over the 1.6 to 250 µg/mL range, replicates at the lowest concentration for most cannabinoids deviated from their mean by >5%, indicating significant baseline noise at this level. Accordingly, calibration curves were constructed using the six standards from 3.1 to 250 µg/mL. The corresponding linear equations and their R2 are summarized in Table 2. Good linearity was obtained, with R2 > 0.9999 for all the analytes. The magnitudes of the intercepts were compared to the integrations at the working standard concentration, and all were deemed insignificant, as 3% of the working standard integration was greater than the absolute value of the intercept. Thus, the calibration equations were linear and passed sufficiently close to the origin for RRF values determined from the working standard concentration to be reliable.
| ||||||||||||||||||||||||||||||||||||||||||||||||
Retention times compared to relative retention times
Retention times pooled from the three analysts are reported in Table 3. The CV in the retention times for each cannabinoid ranged from 0.18% to 0.56%, demonstrating an excellent inter-batch repeatability. For the cannabinoids detected in the available inflorescence samples, the retention times observed for the sample peaks deviated by <1% from the standard retention times.
| ||||||||||||||||||||||||||||||||||||||||||||||||
To formalize the peak identification, and to demonstrate further gains in the inter-batch repeatability, the RRT were also pooled from the three analysts and were appended to Table 3. RRT should correct for inter-batch variations in retention times, provided that the variation in conditions proportionally affected all of the closely related analytes being studied.[27] As anticipated, the pooled RRT values for each cannabinoid had CV ranging from 0.04 to 0.34%. This represents a modest gain in repeatability, which should be maintained even if the retention times start to shift by >1%. Critically, it was also shown that the range of RRT values for each cannabinoid did not overlap. This means that analysts reported comparable values for the RRT, and that these values were unique for each cannabinoid. Thus, cannabinoid peaks in samples may be identified from their RRT values relative to the retention time of the CBD or CBDA from the working standard tested in the same batch of analysis.
Relative response factors
From the same replicate working standard injections performed by the three analysts, the pooled RRF were determined and appended to Table 3. The CV ranged from 1.29 to 2.67%, acceptably within the 3% criteria. Agreement was demonstrated between the RRF values calculated by each analyst. Thus, with an acceptably small error, cannabinoids can be quantified using the RRF values relative to response factors for CBD or CBDA from the working standard tested in the same batch of analysis. This use of RRF for the quantification of selected cannabinoids in cannabis products is a novel contribution of the present study, which eliminates the need for the expensive cannabinoid standards during routine analysis.
Detection and quantification limits
Detection and quantification limits for the cannabinoids are presented in Table 4. The LoD ranged from 20 to 78 µg/g and the LoQ ranged from 60 to 238 µg/g, relative to the inflorescence sample preparation. These limits are sufficiently low to enable the quantification of the studied cannabinoids in cannabis biomass and, observing that even relatively small amounts in crude biomass can be extracted and concentrated to therapeutically relevant concentrations in final products, these limits are suitable for quality control throughout the supply chain. However, with the quantification limits in the determined order of magnitude, it is unlikely that this method could be adapted for the analysis of the recently identified trace cannabinoids with heptyl side-chains (denoted with the suffix "-phoryl").[28] This includes Δ9-trans-Tetrahydrocannabiphorol (THCP), which, by a published MS method, was identified in the inflorescences of THC dominant chemovars at concentrations routinely less than 140 µg/g and was undetected in CBD dominant chemovars.[35]
| ||||||||||||||||||||||||||||||||||||
Instrument, method, and intermediate precision
Instrument and method precision results are collated in Table 5. Instrument precision ranged from 0.10 to 2.00%, which was acceptably below 2%. Method precision ranged from 1.15 to 3.58% for the inflorescence and from 1.27 to 1.32% for the oil, acceptably within 5% for both sample matrices. Concentrations reported by the independent analysts for the samples tested on separate days and the intermediate precision were reported in Table 6. The results of the analysts agreed, with the intermediate precision ranging from 0.67 to 4.58% for the inflorescence and from 1.28 to 1.60% for the oil. Though two of the analysts had no prior exposure to the method, an acceptable precision of <5% was achieved for both sample matrices, thus demonstrating the robustness of the method.
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Accuracy and recovery
Accuracy of the method was evaluated from the recoveries of analytes spiked onto surrogate matrices, as presented in Table 7. For the cannabis inflorescence and oil, the spike recoveries from the surrogate matrices ranged from 90.1 to 109.3% (mean 100.9%) and from 95.4 to 103.1% (mean 99.6%), respectively. Most recoveries were within 5% of the nominal concentration and the only two recoveries which were outside of this criterion had been spiked at the quantification limit, so their recoveries within 10% were acceptable. The precision of the recoveries was also acceptable, except at the LoQ of Δ8-THC and CBDV, which were only precise to 12%. Therefore, the method for the quantification of cannabinoids has acceptable accuracy.
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
` In this study, chamomile was selected as a surrogate matrix for cannabis inflorescence as it was floral, available at little cost, and, with the exception of the cannabinoids, shared phytochemical classes such as fragrant terpenes and flavonoids.[10][36] Other published articles have used Urtica dioica (stinging nettle)[37][38] or Humulus lupulus (beer hops)[24], with justifications based on tracing their phylogenies relative to Cannabis sativa. Whilst sharing botanical orders or even families does not necessarily provide better matrix matching, it may be a reasonable approximation. Likewise, for cannabis oil, the choice of olive oil as a surrogate matrix had precedent from previous publications.[39] Indeed, some cannabis oil products contain refined resins or even crude inflorescence extracted into an olive oil base[40], making its choice as the surrogate matrix reasonable for such products. The appearance of publications employing surrogate matrices is being increasingly accepted as a cost-reduction strategy during method development, which is a clear advantage over articles which did not conduct recovery studies at all.[41][42][43] Analysts in some jurisdictions may also find it pertinent to consider the use of surrogate matrices if licensing requirements preclude the use of the amount of cannabis material which would be required for the complete spike-recovery protocol on the true matrices.
Standard and sample stability
Response factors were determined from six replicate injections of a freshly diluted CBD and CBDA working standard. The standards were stored for 24 hours in a resealing vial within the autosampler at 10 °C. Subsequently, another six injections were made, and the response factors from the original determination were used to calculate that CBD and CBDA were 101.5% and 98.1% of their original concentrations, respectively. Observing that the changes in concentration were less than the 2% criteria used to validate instrument precision, the working standard was deemed stable. The stability of cannabinoids in the cannabis oil extract was evaluated at 48 hours. At this time point, Δ9-THC and CBD were 98.4% and 97.3% of their original concentrations, respectively. Accordingly, compared to the 5% criteria utilized to validate the method precision, the oil sample extracts were deemed stable. This extended stability relieves the pressure on laboratories to analyze the samples quickly after extraction.
Comparing quantification methods
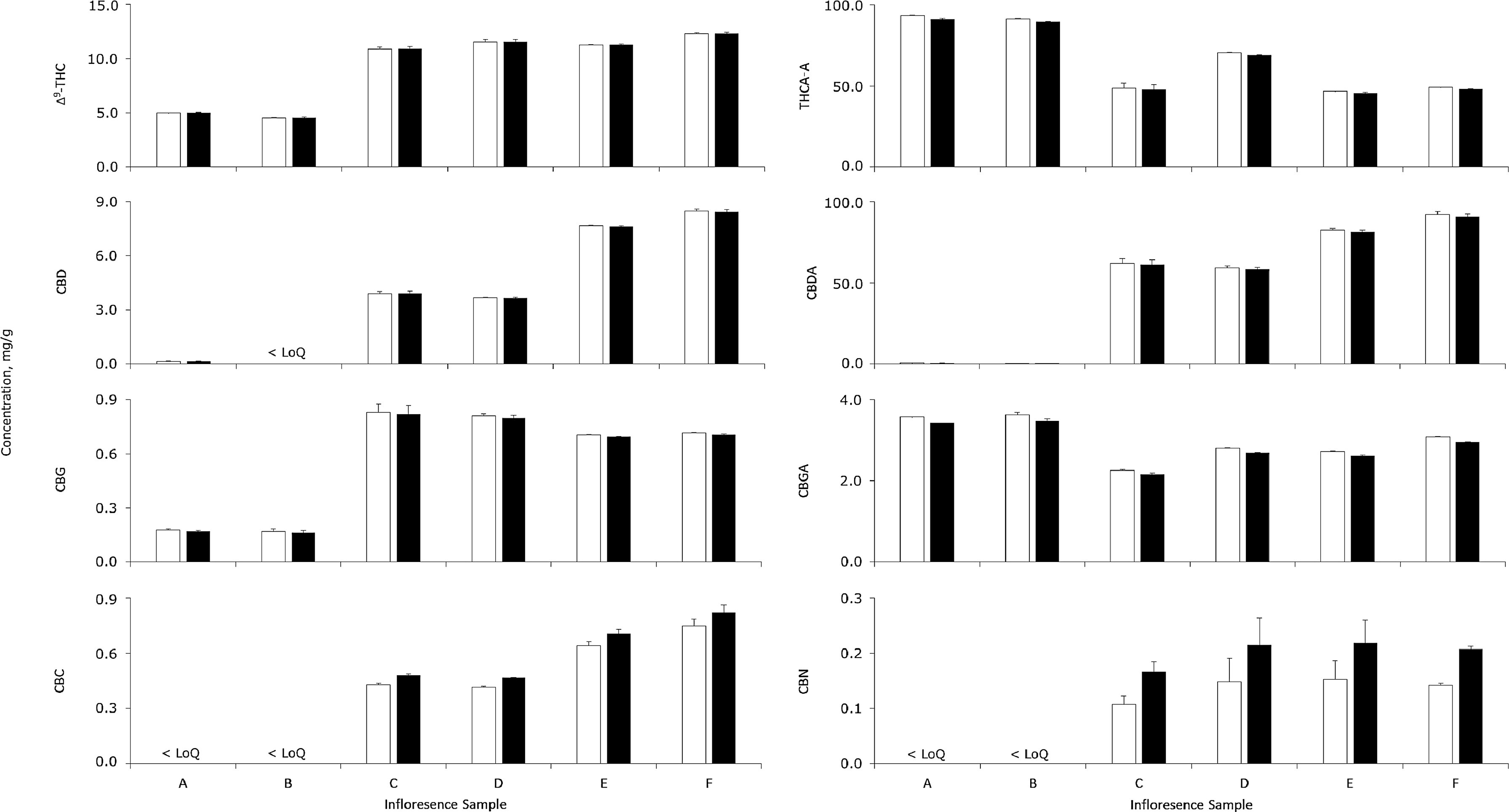
Cannabinoid concentrations in six different inflorescence samples were determined by conventional multipoint calibrations and the RRF method, as reported in Fig. 3. For cannabinoids above the order of magnitude of the LoQ, concentrations determined by the two methods agreed satisfactorily (range, 95.0 to 111.9%; mean, 100.0%). The only cannabinoid above the LoQ which differed between quantifications by more than 5% was CBC but, relative to its low concentrations, the absolute differences was always acceptably less than 80 µg/g. The good agreement between the results obtained using the two different quantification methods applied to real samples demonstrates that the use of RRF for quantification is a valid alternative with its concomitant cost saving.
|
Considering the cannabinoid profiles of the inflorescence samples, the high ratios of acidic to neutral cannabinoids were indicative of good drying and storage conditions. Furthermore, samples A and B were classified as having moderately high total THC (∼100 mg/g) and low total CBD (<1 mg/g), whilst samples C to F had moderate amounts of both (∼60 to 90 mg/g). Beyond these observed concentrations, the proposed method is appropriate to analyse most samples with even greater levels of cannabinoids, as very few inflorescences exceed 200 mg/g total THC.[34] Other cannabinoids such as CBC and CBN were also quantifiable, but Δ8-THC was not detected in any sample. However, other authors have reportedly identified inflorescence samples with Δ8-THC concentrations up to 4.9 mg/g[44], well above the LoQ of the present method. Accordingly, the present method has sufficient dynamic range to quantify cannabinoids at their various native concentrations.
Conclusion
A simple HPLC-PDA method has been developed for the analysis of Δ9-THC, Δ8-THC, THCA-A, CBD, CBDA, CBG, CBGA, CBN, CBDV, and CBC in the inflorescence and oil of medicinal cannabis. This method was validated according to ICH guidelines. During the validation process, surrogate matrices were shown to be viable substitutions when costs prohibit the required replicates for spiking onto the true matrices. Considering the RRT and RRF values, they were consistent between batches independently performed by three analysts. Moreover, the validity of using RRT and RRF was demonstrated as the quantifications of cannabinoids in six inflorescence samples agreed with the conventional approach of multipoint calibration.
Collectively, analysts in the medicinal cannabis field are encouraged to examine this method and considerate for use. Before use, analysts need to validate the RRF quantification for their existing methods and for any new methods that they design; potentially including methods capable of analyzing broader panels than the 10-cannabinoid panel tested in this work. In doing so, cost barriers for the analysis of panels of cannabinoids can be overcome, such that a diversity of cannabinoids can be analyzed as a part of routine quality control, with results that reflect the therapeutic efficacy for the consumer.
Abbreviations, acronyms, and initialisms
- Δ8-THC: delta-8-Tetrahydrocannabinol
- Δ9-THC: tetrahydrocannabinol
- CBC: cannabichromene
- CBD: cannabidiol
- CBDA: cannabidiolic acid
- CBDV: cannabidivarin
- CBG: cannabigerol
- CBGA: cannabigerolic acid
- CBN: cannabinol
- CV: coefficient of variation
- GC: gas chromatography
- HPLC-PDA: high-performance liquid chromatography-photodiode array
- ICH: International Council for Harmonisation
- LC: liquid chromatography
- LoD: limit of detection
- LoQ: limit of quantitation
- MS: mass spectrometry
- PDA: photodiode array
- QC: quality control
- RF: response factor
- RRF: relative response factor
- RRT: relative retention time
- THCA-A: delta-9-Tetrahydrocannabinolic acid A
- THCP: delta-9-trans-Tetrahydrocannabiphorol
- THCV: tetrahydrocannabivarin
- tR: retention time
- UV-Vis: ultraviolet–visible spectroscopy
Acknowledgements
The authors wish to thank Little Green Pharma and Health House International for supplying products to assist with this method development project.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interest
None stated.
References
- ↑ Zuardi, Antonio Waldo (1 June 2006). "History of cannabis as a medicine: a review". Revista Brasileira de Psiquiatria 28 (2): 153–157. doi:10.1590/S1516-44462006000200015. ISSN 1516-4446. http://www.scielo.br/scielo.php?script=sci_arttext&pid=S1516-44462006000200015&lng=en&tlng=en.
- ↑ Guy, Geoffrey W.; Whittle, B. A.; Robson, Philip, eds. (2004). The medicinal uses of cannabis and cannabinoids. London ; Chicago: Pharmaceutical Press. pp. 1–16. ISBN 978-0-85369-517-2. OCLC ocm56593404. https://www.worldcat.org/title/mediawiki/oclc/ocm56593404.
- ↑ Russo, Ethan B. (21 August 2007). "History of Cannabis and Its Preparations in Saga, Science, and Sobriquet" (in en). Chemistry & Biodiversity 4 (8): 1614–1648. doi:10.1002/cbdv.200790144. https://onlinelibrary.wiley.com/doi/10.1002/cbdv.200790144.
- ↑ Musto, David F. (1 February 1972). "The Marihuana Tax Act of 1937" (in en). Archives of General Psychiatry 26 (2): 101–8. doi:10.1001/archpsyc.1972.01750200005002. ISSN 0003-990X. http://archpsyc.jamanetwork.com/article.aspx?doi=10.1001/archpsyc.1972.01750200005002.
- ↑ Ferraiolo, Kathleen (1 April 2007). "From Killer Weed to Popular Medicine: The Evolution of American Drug Control Policy, 1937–2000" (in en). Journal of Policy History 19 (2): 147–179. doi:10.1353/jph.2007.0009. ISSN 0898-0306. https://www.cambridge.org/core/product/identifier/S0898030600001615/type/journal_article.
- ↑ 6.0 6.1 National Academies of Sciences, Engineering, and Medicine (U.S.), ed. (2017). The health effects of cannabis and cannabinoids: the current state of evidence and recommendations for research. Washington, DC: The National Academies Press. ISBN 978-0-309-45304-2. OCLC 984512600. https://www.worldcat.org/title/mediawiki/oclc/984512600.
- ↑ Press, Craig A.; Knupp, Kelly G.; Chapman, Kevin E. (1 April 2015). "Parental reporting of response to oral cannabis extracts for treatment of refractory epilepsy" (in en). Epilepsy & Behavior 45: 49–52. doi:10.1016/j.yebeh.2015.02.043. https://linkinghub.elsevier.com/retrieve/pii/S1525505015001043.
- ↑ Suraev, A.; Lintzeris, N.; Stuart, J.; Kevin, R. C.; Blackburn, R.; Richards, E.; Arnold, J. C.; Ireland, C. et al. (1 December 2018). "Composition and Use of Cannabis Extracts for Childhood Epilepsy in the Australian Community" (in en). Scientific Reports 8 (1): 10154. doi:10.1038/s41598-018-28127-0. ISSN 2045-2322. PMC PMC6033872. PMID 29977078. http://www.nature.com/articles/s41598-018-28127-0.
- ↑ Treat, Lauren; Chapman, Kevin E.; Colborn, Kathryn L.; Knupp, Kelly G. (1 January 2017). "Duration of use of oral cannabis extract in a cohort of pediatric epilepsy patients" (in en). Epilepsia 58 (1): 123–127. doi:10.1111/epi.13617. https://onlinelibrary.wiley.com/doi/10.1111/epi.13617.
- ↑ 10.0 10.1 Gonçalves, Joana; Rosado, Tiago; Soares, Sofia; Simão, Ana; Caramelo, Débora; Luís, Ângelo; Fernández, Nicolás; Barroso, Mário et al. (23 February 2019). "Cannabis and Its Secondary Metabolites: Their Use as Therapeutic Drugs, Toxicological Aspects, and Analytical Determination" (in en). Medicines 6 (1): 31. doi:10.3390/medicines6010031. ISSN 2305-6320. PMC PMC6473697. PMID 30813390. http://www.mdpi.com/2305-6320/6/1/31.
- ↑ Hanuš, Lumír Ondřej; Meyer, Stefan Martin; Muñoz, Eduardo; Taglialatela-Scafati, Orazio; Appendino, Giovanni (2016). "Phytocannabinoids: a unified critical inventory" (in en). Natural Product Reports 33 (12): 1357–1392. doi:10.1039/C6NP00074F. ISSN 0265-0568. http://xlink.rsc.org/?DOI=C6NP00074F.
- ↑ Upton, R.; Craker, L.; ElSohly, M. et al. (2013). American Herbal Pharmacopeia: Cannabis Inflorescence. American Herbal Pharmacopeia. ISBN 1929425333.
- ↑ Anderson, Lyndsey L.; Low, Ivan K.; Banister, Samuel D.; McGregor, Iain S.; Arnold, Jonathon C. (22 November 2019). "Pharmacokinetics of Phytocannabinoid Acids and Anticonvulsant Effect of Cannabidiolic Acid in a Mouse Model of Dravet Syndrome" (in en). Journal of Natural Products 82 (11): 3047–3055. doi:10.1021/acs.jnatprod.9b00600. ISSN 0163-3864. https://pubs.acs.org/doi/10.1021/acs.jnatprod.9b00600.
- ↑ Ben-Shabat, Shimon; Fride, Ester; Sheskin, Tzviel; Tamiri, Tsippy; Rhee, Man-Hee; Vogel, Zvi; Bisogno, Tiziana; De Petrocellis, Luciano et al. (1 July 1998). "An entourage effect: inactive endogenous fatty acid glycerol esters enhance 2-arachidonoyl-glycerol cannabinoid activity" (in en). European Journal of Pharmacology 353 (1): 23–31. doi:10.1016/S0014-2999(98)00392-6. https://linkinghub.elsevier.com/retrieve/pii/S0014299998003926.
- ↑ McPartland, John M.; Russo, Ethan B. (1 June 2001). "Cannabis and Cannabis Extracts: Greater Than the Sum of Their Parts?" (in en). Journal of Cannabis Therapeutics 1 (3-4): 103–132. doi:10.1300/J175v01n03_08. ISSN 1529-9775. http://www.tandfonline.com/doi/abs/10.1300/J175v01n03_08.
- ↑ Anderson, Lyndsey L.; Etchart, Maia G.; Bahceci, Dilara; Golembiewski, Taliesin A.; Arnold, Jonathon C. (22 July 2021). "Cannabis constituents interact at the drug efflux pump BCRP to markedly increase plasma cannabidiolic acid concentrations" (in en). Scientific Reports 11 (1): 14948. doi:10.1038/s41598-021-94212-6. ISSN 2045-2322. PMC PMC8298633. PMID 34294753. https://www.nature.com/articles/s41598-021-94212-6.
- ↑ Goerl, Brett; Watkins, Sarah; Metcalf, Cameron; Smith, Misty; Beenhakker, Mark (1 January 2021). "Cannabidiolic acid exhibits entourage-like improvements of anticonvulsant activity in an acute rat model of seizures" (in en). Epilepsy Research 169: 106525. doi:10.1016/j.eplepsyres.2020.106525. PMC PMC7855831. PMID 33310415. https://linkinghub.elsevier.com/retrieve/pii/S0920121120305763.
- ↑ Hill, T D M; Cascio, M-G; Romano, B; Duncan, M; Pertwee, R G; Williams, C M; Whalley, B J; Hill, A J (1 October 2013). "Cannabidivarin-rich cannabis extracts are anticonvulsant in mouse and rat via a CB 1 receptor-independent mechanism: CBDV-rich extracts are anticonvulsant" (in en). British Journal of Pharmacology 170 (3): 679–692. doi:10.1111/bph.12321. PMC PMC3792005. PMID 23902406. https://onlinelibrary.wiley.com/doi/10.1111/bph.12321.
- ↑ Samarut, Éric; Nixon, Jessica; Kundap, Uday P.; Drapeau, Pierre; Ellis, Lee D. (20 March 2019). "Single and Synergistic Effects of Cannabidiol and Δ-9-Tetrahydrocannabinol on Zebrafish Models of Neuro-Hyperactivity". Frontiers in Pharmacology 10: 226. doi:10.3389/fphar.2019.00226. ISSN 1663-9812. PMC PMC6435997. PMID 30949046. https://www.frontiersin.org/article/10.3389/fphar.2019.00226/full.
- ↑ Casey, Sherelle L.; Atwal, Nicholas; Vaughan, Christopher W. (1 December 2017). "Cannabis constituent synergy in a mouse neuropathic pain model" (in en). Pain 158 (12): 2452–2460. doi:10.1097/j.pain.0000000000001051. ISSN 0304-3959. https://journals.lww.com/00006396-201712000-00019.
- ↑ King, Kirsten M; Myers, Alyssa M; Soroka-Monzo, Ariele J; Tuma, Ronald F; Tallarida, Ronald J; Walker, Ellen A; Ward, Sara Jane (1 September 2017). "Single and combined effects of Δ 9 -tetrahydrocannabinol and cannabidiol in a mouse model of chemotherapy-induced neuropathic pain: THC and CBD synergistically attenuate neuropathic pain" (in en). British Journal of Pharmacology 174 (17): 2832–2841. doi:10.1111/bph.13887. PMC PMC5554313. PMID 28548225. https://onlinelibrary.wiley.com/doi/10.1111/bph.13887.
- ↑ Pamplona, Fabricio A.; da Silva, Lorenzo Rolim; Coan, Ana Carolina (12 September 2018). "Potential Clinical Benefits of CBD-Rich Cannabis Extracts Over Purified CBD in Treatment-Resistant Epilepsy: Observational Data Meta-analysis". Frontiers in Neurology 9: 759. doi:10.3389/fneur.2018.00759. ISSN 1664-2295. PMC PMC6143706. PMID 30258398. https://www.frontiersin.org/article/10.3389/fneur.2018.00759/full.
- ↑ Mehmedic, Zlatko; Chandra, Suman; Slade, Desmond; Denham, Heather; Foster, Susan; Patel, Amit S.; Ross, Samir A.; Khan, Ikhlas A. et al. (1 September 2010). "Potency Trends of Δ9-THC and Other Cannabinoids in Confiscated Cannabis Preparations from 1993 to 2008*: POTENCY TRENDS OF Δ9-THC (1993-2008)" (in en). Journal of Forensic Sciences 55 (5): 1209–1217. doi:10.1111/j.1556-4029.2010.01441.x. https://onlinelibrary.wiley.com/doi/10.1111/j.1556-4029.2010.01441.x.
- ↑ 24.0 24.1 24.2 24.3 24.4 Leghissa, Allegra; Hildenbrand, Zacariah L.; Foss, Frank W.; Schug, Kevin A. (1 January 2018). "Determination of cannabinoids from a surrogate hops matrix using multiple reaction monitoring gas chromatography with triple quadrupole mass spectrometry" (in en). Journal of Separation Science 41 (2): 459–468. doi:10.1002/jssc.201700946. https://onlinelibrary.wiley.com/doi/10.1002/jssc.201700946.
- ↑ 25.0 25.1 25.2 Ibrahim, Elsayed; Gul, Waseem; Gul, Shahbaz; Stamper, Brandon; Hadad, Ghada; Abdel Salam, Randa; Ibrahim, Amany; Ahmed, Safwat et al. (1 March 2018). "Determination of Acid and Neutral Cannabinoids in Extracts of Different Strains of Cannabis sativa Using GC-FID" (in en). Planta Medica 84 (04): 250–259. doi:10.1055/s-0043-124088. ISSN 0032-0943. http://www.thieme-connect.de/DOI/DOI?10.1055/s-0043-124088.
- ↑ Berman, Paula; Futoran, Kate; Lewitus, Gil M.; Mukha, Dzmitry; Benami, Maya; Shlomi, Tomer; Meiri, David (1 December 2018). "A new ESI-LC/MS approach for comprehensive metabolic profiling of phytocannabinoids in Cannabis" (in en). Scientific Reports 8 (1): 14280. doi:10.1038/s41598-018-32651-4. ISSN 2045-2322. PMC PMC6155167. PMID 30250104. http://www.nature.com/articles/s41598-018-32651-4.
- ↑ 27.0 27.1 Hazekamp, Arno; Peltenburg, Anja; Verpoorte, Rob; Giroud, Christian (1 September 2005). "Chromatographic and Spectroscopic Data of Cannabinoids from Cannabis sativa L." (in en). Journal of Liquid Chromatography & Related Technologies 28 (15): 2361–2382. doi:10.1080/10826070500187558. ISSN 1082-6076. https://www.tandfonline.com/doi/full/10.1080/10826070500187558.
- ↑ 28.0 28.1 Citti, Cinzia; Linciano, Pasquale; Russo, Fabiana; Luongo, Livio; Iannotta, Monica; Maione, Sabatino; Laganà, Aldo; Capriotti, Anna Laura et al. (1 December 2019). "A novel phytocannabinoid isolated from Cannabis sativa L. with an in vivo cannabimimetic activity higher than Δ9-tetrahydrocannabinol: Δ9-Tetrahydrocannabiphorol" (in en). Scientific Reports 9 (1): 20335. doi:10.1038/s41598-019-56785-1. ISSN 2045-2322. PMC PMC6937300. PMID 31889124. http://www.nature.com/articles/s41598-019-56785-1.
- ↑ Linciano, Pasquale; Russo, Fabiana; Citti, Cinzia; Tolomeo, Francesco; Paris, Roberta; Fulvio, Flavia; Pecchioni, Nicola; Vandelli, Maria Angela et al. (1 December 2021). "The novel heptyl phorolic acid cannabinoids content in different Cannabis sativa L. accessions" (in en). Talanta 235: 122704. doi:10.1016/j.talanta.2021.122704. https://linkinghub.elsevier.com/retrieve/pii/S0039914021006251.
- ↑ International Conference for Harmonisation (2005). "Validation of analytical procedures: Text and methodology" (PDF). https://database.ich.org/sites/default/files/Q2(R1)%20Guideline.pdf. Retrieved 12 October 2021.
- ↑ Wang, Yan-Hong; Avula, Bharathi; ElSohly, Mahmoud; Radwan, Mohamed; Wang, Mei; Wanas, Amira; Mehmedic, Zlatko; Khan, Ikhlas (1 March 2018). "Quantitative Determination of Δ9-THC, CBG, CBD, Their Acid Precursors and Five Other Neutral Cannabinoids by UHPLC-UV-MS" (in en). Planta Medica 84 (04): 260–266. doi:10.1055/s-0043-124873. ISSN 0032-0943. http://www.thieme-connect.de/DOI/DOI?10.1055/s-0043-124873.
- ↑ Nahar, Lutfun; Guo, Mingquan; Sarker, Satyajit D. (1 March 2020). "Gas chromatographic analysis of naturally occurring cannabinoids: A review of literature published during the past decade" (in en). Phytochemical Analysis 31 (2): 135–146. doi:10.1002/pca.2886. ISSN 0958-0344. https://onlinelibrary.wiley.com/doi/10.1002/pca.2886.
- ↑ Ciolino, Laura A.; Ranieri, Tracy L.; Taylor, Allison M. (1 August 2018). "Commercial cannabis consumer products part 2: HPLC-DAD quantitative analysis of cannabis cannabinoids" (in en). Forensic Science International 289: 438–447. doi:10.1016/j.forsciint.2018.05.033. https://linkinghub.elsevier.com/retrieve/pii/S0379073818302858.
- ↑ 34.0 34.1 Taschwer, Magdalena; Schmid, Martin G. (1 September 2015). "Determination of the relative percentage distribution of THCA and Δ9-THC in herbal cannabis seized in Austria – Impact of different storage temperatures on stability" (in en). Forensic Science International 254: 167–171. doi:10.1016/j.forsciint.2015.07.019. https://linkinghub.elsevier.com/retrieve/pii/S0379073815002972.
- ↑ Bueno, Justin; Greenbaum, Eric A. (26 February 2021). "(−)- trans -Δ 9 -Tetrahydrocannabiphorol Content of Cannabis sativa Inflorescence from Various Chemotypes" (in en). Journal of Natural Products 84 (2): 531–536. doi:10.1021/acs.jnatprod.0c01034. ISSN 0163-3864. https://pubs.acs.org/doi/10.1021/acs.jnatprod.0c01034.
- ↑ Singh, Ompal; Khanam, Zakia; Misra, Neelam; Srivastava, ManojKumar (2011). "Chamomile (Matricaria chamomilla L.): An overview" (in en). Pharmacognosy Reviews 5 (9): 82. doi:10.4103/0973-7847.79103. ISSN 0973-7847. PMC PMC3210003. PMID 22096322. http://www.phcogrev.com/article/2011/5/9/1041030973-784779103.
- ↑ De Backer, Benjamin; Debrus, Benjamin; Lebrun, Pierre; Theunis, Laetitia; Dubois, Nathalie; Decock, Lies; Verstraete, Alain; Hubert, Philippe et al. (1 December 2009). "Innovative development and validation of an HPLC/DAD method for the qualitative and quantitative determination of major cannabinoids in cannabis plant material" (in en). Journal of Chromatography B 877 (32): 4115–4124. doi:10.1016/j.jchromb.2009.11.004. https://linkinghub.elsevier.com/retrieve/pii/S1570023209007545.
- ↑ Mudge, Elizabeth M.; Murch, Susan J.; Brown, Paula N. (1 May 2017). "Leaner and greener analysis of cannabinoids" (in en). Analytical and Bioanalytical Chemistry 409 (12): 3153–3163. doi:10.1007/s00216-017-0256-3. ISSN 1618-2642. PMC PMC5395585. PMID 28233028. http://link.springer.com/10.1007/s00216-017-0256-3.
- ↑ Madej, Katarzyna; Kózka, Gabriela; Winiarski, Maciej; Piekoszewski, Wojciech (29 October 2020). "A Simple, Fast, and Green Oil Sample Preparation Method for Determination of Cannabidioloic Acid and Cannabidiol by HPLC-DAD" (in en). Separations 7 (4): 60. doi:10.3390/separations7040060. ISSN 2297-8739. https://www.mdpi.com/2297-8739/7/4/60.
- ↑ Citti, Cinzia; Ciccarella, Giuseppe; Braghiroli, Daniela; Parenti, Carlo; Vandelli, Maria Angela; Cannazza, Giuseppe (1 September 2016). "Medicinal cannabis: Principal cannabinoids concentration and their stability evaluated by a high performance liquid chromatography coupled to diode array and quadrupole time of flight mass spectrometry method" (in en). Journal of Pharmaceutical and Biomedical Analysis 128: 201–209. doi:10.1016/j.jpba.2016.05.033. https://linkinghub.elsevier.com/retrieve/pii/S0731708516302722.
- ↑ Ingallina, Cinzia; Sobolev, Anatoly P.; Circi, Simone; Spano, Mattia; Fraschetti, Caterina; Filippi, Antonello; Di Sotto, Antonella; Di Giacomo, Silvia et al. (20 April 2020). "Cannabis sativa L. Inflorescences from Monoecious Cultivars Grown in Central Italy: An Untargeted Chemical Characterization from Early Flowering to Ripening" (in en). Molecules 25 (8): 1908. doi:10.3390/molecules25081908. ISSN 1420-3049. PMC PMC7221798. PMID 32326129. https://www.mdpi.com/1420-3049/25/8/1908.
- ↑ Ambach, Lars; Penitschka, Franziska; Broillet, Alain; König, Stefan; Weinmann, Wolfgang; Bernhard, Werner (1 October 2014). "Simultaneous quantification of delta-9-THC, THC-acid A, CBN and CBD in seized drugs using HPLC-DAD" (in en). Forensic Science International 243: 107–111. doi:10.1016/j.forsciint.2014.06.008. https://linkinghub.elsevier.com/retrieve/pii/S0379073814002448.
- ↑ Elkins, Aaron C.; Deseo, Myrna A.; Rochfort, Simone; Ezernieks, Vilnis; Spangenberg, German (1 March 2019). "Development of a validated method for the qualitative and quantitative analysis of cannabinoids in plant biomass and medicinal cannabis resin extracts obtained by super-critical fluid extraction" (in en). Journal of Chromatography B 1109: 76–83. doi:10.1016/j.jchromb.2019.01.027. https://linkinghub.elsevier.com/retrieve/pii/S1570023218318257.
- ↑ Gul, Waseem; Gul, Shahbaz W; Radwan, Mohamed M; Wanas, Amira S; Mehmedic, Zlatko; Khan, Ikhlas I; Sharaf, Maged H M; ElSohly, Mahmoud A (1 November 2015). "Determination of 11 Cannabinoids in Biomass and Extracts of Different Varieties of Cannabis Using High-Performance Liquid Chromatography" (in en). Journal of AOAC INTERNATIONAL 98 (6): 1523–1528. doi:10.5740/jaoacint.15-095. ISSN 1060-3271. https://academic.oup.com/jaoac/article/98/6/1523/5654536.
Notes
This presentation is faithful to the original, with only a few minor changes to presentation. Some grammar and punctuation was cleaned up to improve readability. In some cases important information was missing from the references, and that information was added. One of the sentences in the original's conclusion was incomplete ("analysts in the medicinal cannabis field are encouraged to"); for this version, a reasonable presumption was made towards how that sentence should have ended.