Difference between revisions of "Journal:Evaluating the effectiveness of a new student-centred laboratory training strategy in clinical biochemistry teaching"
Shawndouglas (talk | contribs) (Saving and adding more.) |
Shawndouglas (talk | contribs) (Saving and adding more.) |
||
| Line 56: | Line 56: | ||
====Test group==== | ====Test group==== | ||
The laboratory training adopted a new student-centered training program that was divided into four stages. First, students had access to the case (with the questions) at least two to four days before the class and were asked to answer several basic questions individually about the case before the class, e.g., what the diagnosis is based on, what the detection indicators are, and what the the indicators of a certain inspection procedures for pre-analysis, analysis, and post-analysis are. The answers of each minor group were then shared in the class, and the students tried to reach a consensus among the groups, with the teachers’ facilitation. This stage took approximately 30 minutes. Second, it took 30 minutes to learn principles, which was mainly an explanation of the current commonly used methods and principles. Third, it took 45 minutes to improve their lab skills, including the evaluation of lab conditions, assessment of equipment conditions, use of internal control, and sample processing according to the standard operating procedure (SOP). Fourth, results were analyzed by combining the ISO 15189 requirements with the teaching contents to improve the operations in 30 minutes. The main concern was the review and reporting of results. When abnormal or suspicious results occurred, the students were able to identify them. The teachers facilitated the entire process. If the results were not judged correctly, the teacher asked students to re-check the result until they met the re-inspection requirements, and the students analyzed whether the results could be issued. After that, the students were asked to conduct a quiz and an after-class survey. | |||
===Control group=== | |||
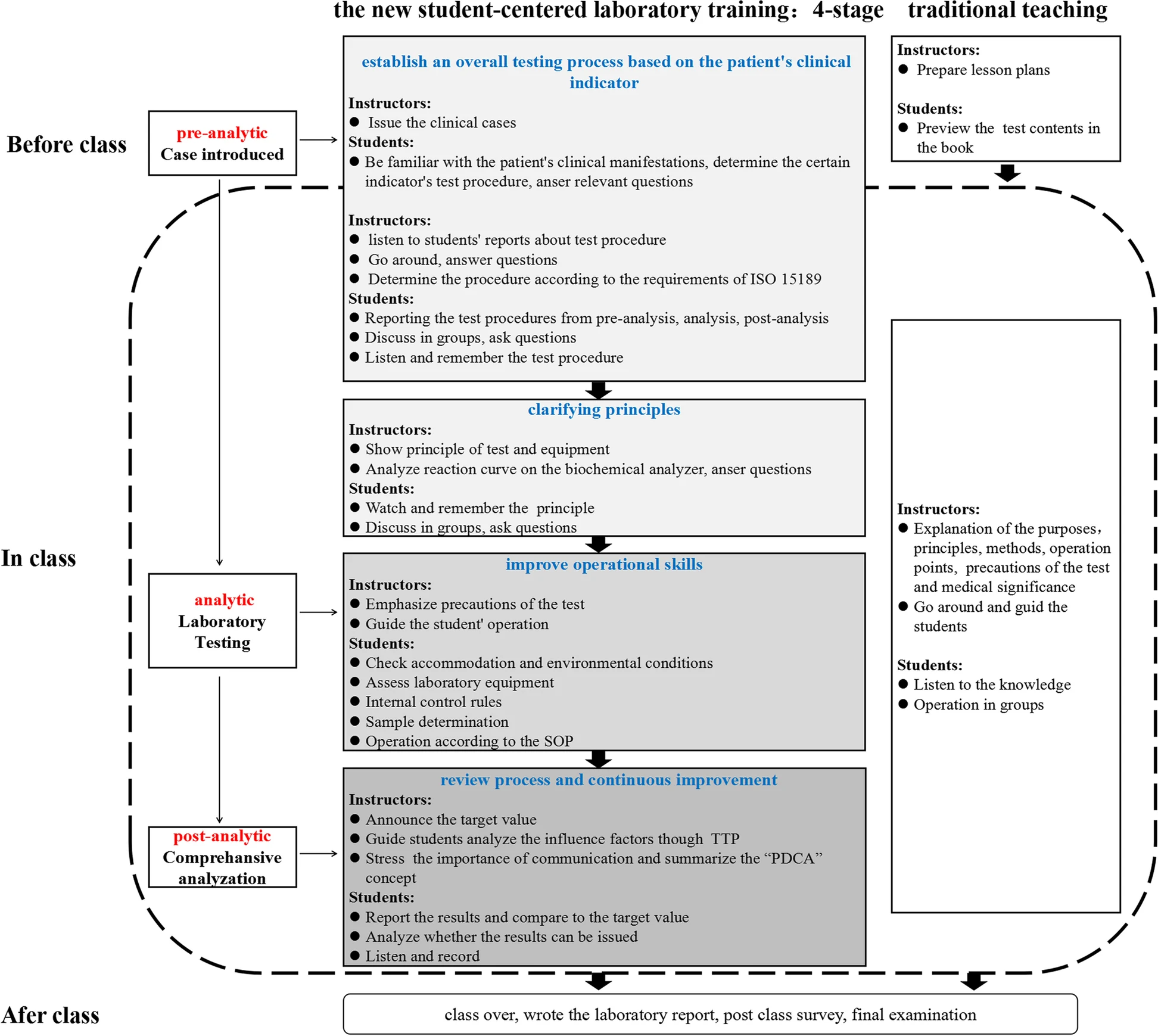
The knowledge and theoretical outline of the clinical biochemistry course in the lectures was the same as that of the test group. Experimental teaching was implemented in a teacher-centered way. The teacher explained the principles, operation points, and medical significance, and then the students performed the experiment. A schematic diagram of the teaching mode between the two groups is shown in Figure 1. | |||
[[File:Fig1 Xu BMCMedEd23 23.png|1000px]] | |||
{{clear}} | |||
{| | |||
| style="vertical-align:top;" | | |||
{| border="0" cellpadding="5" cellspacing="0" width="1000px" | |||
|- | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |<blockquote>'''Fig. 1''' Schematic diagram of the pathway comparison between the new student-centred laboratory training program and the traditional training program in clinical biochemistry. SOP: Standard Operating Procedure; TTP: Total Testing Process; PDCA: Plan, Do, Check, Act.</blockquote> | |||
|- | |||
|} | |||
|} | |||
Here is an example. Students became familiar with the clinical manifestations of a patient with recurrent systemic edema (finally diagnosed as nephrotic syndrome) two weeks before the class on that same topic. When the nephrotic syndrome was diagnosed, urinalysis, blood counts and coagulation panel, renal function and electrolytes, liver panel, and glucose tests were required. Students were asked to report why and how the test procedure for “creatine and urea” in renal function was determined. Finally, students were then asked to discuss the examination process and medical significance, and analyze various factors that may affect the test result, including pre-pre-analysis (i.e., test selection, test ordering, patient/specimen identification), pre-analysis (i.e., specimen collection, transportation, specimen processing, specimen preparation), analytic, post-analysis (i.e., report review, result reporting), and post-post-analysis (i.e., result interpretation) in accordance with the requirements of ISO 15189. [21] Then students made an operation plan according to the inspection process of the project, and the teachers evaluated and determined the testing procedure. | |||
===Outcome evaluation=== | |||
====Assessment for laboratory operation==== | |||
To evaluate students’ students’ awareness of and ability to use quality management practices in TTP and other laboratory processes, evaluation indicators were designed as shown in Table 1. | |||
{| | |||
| style="vertical-align:top;" | | |||
{| class="wikitable" border="1" cellpadding="5" cellspacing="0" width="100%" | |||
|- | |||
| colspan="6" style="background-color:white; padding-left:10px; padding-right:10px;" |'''Table 1.''' The evaluation system of the experimental operation. | |||
|- | |||
! style="background-color:#dddddd; padding-left:10px; padding-right:10px;" colspan="2"|Phase | |||
! style="background-color:#dddddd; padding-left:10px; padding-right:10px;" |Detailed rules of evaluation index | |||
! style="background-color:#dddddd; padding-left:10px; padding-right:10px;" |Grade weights and within area | |||
! style="background-color:#dddddd; padding-left:10px; padding-right:10px;" |Percentages of total | |||
! style="background-color:#dddddd; padding-left:10px; padding-right:10px;" |Evaluation mode | |||
|- | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" rowspan="4"|1 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" rowspan="4"|Pre-analytical | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |* Familiarity with the clinical significance | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |10% | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" rowspan="4"|40% | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |Oral test | |||
|- | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |* Familiarity with the clinical significance | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |10% | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |Oral test | |||
|- | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |* Principle of test indicator | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |10% | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |Paper test | |||
|- | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |* Parameter setting according to the instructions of the kit | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |10% | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |Observation | |||
|- | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" rowspan="6"|2 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" rowspan="6"|Analytical | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |* Check accommodation and environmental conditions, assess laboratory equipment | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |5% | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" rowspan="6"|40% | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |Observation | |||
|- | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |* Use of internal quality control rules | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |5% | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |Oral test | |||
|- | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |* Sample determination | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |10% | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |Oral test | |||
|- | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |* Sample and reagent addition using the pipettes and micro-pipettors | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |10% | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |Observation | |||
|- | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |* Records to be legible | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |5% | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |Observation | |||
|- | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |* Instrument maintenance | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |5% | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |Observation | |||
|- | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" rowspan="3"|3 | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" rowspan="3"|Post-analytical | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |* Results reporting | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |5% | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" rowspan="3"|20% | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |Paper test | |||
|- | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |* Results analysis and judgement: evaluate them in conformity with clinical information available regarding patient | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |10% | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |Paper test | |||
|- | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |* Communication with doctors | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |5% | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |Oral test | |||
|- | |||
|} | |||
|} | |||
| Line 64: | Line 159: | ||
==Notes== | ==Notes== | ||
This presentation is faithful to the original, with only a few minor changes to presentation, spelling, and grammar. In some cases important information was missing from the references, and that information was added | This presentation is faithful to the original, with only a few minor changes to presentation, spelling, and grammar. In some cases important information was missing from the references, and that information was added. | ||
<!--Place all category tags here--> | <!--Place all category tags here--> | ||
Revision as of 23:16, 2 October 2023
| Full article title | Evaluating the effectiveness of a new student-centred laboratory training strategy in clinical biochemistry teaching |
|---|---|
| Journal | BMC Medical Education |
| Author(s) | Xu, Guoying; Zhao, Chuanxiang; Yan, Mengdan; Zhang, Xiaoxian; Zhu, Ling; Liu, Jiaxiu; Zhao, Yaping; Zhang, Yuling; Cai, Weili; Xie, Hongxiang; Jiang, Yuzhang; Shao, Qixiang |
| Author affiliation(s) | Jiangsu College of Nursing, Youyang Medical Laboratory Co., Hangzhou Medical College, Nanjing Medical University |
| Primary contact | Email: shao underscore qx at jscn dot edu dot cn |
| Year published | 2023 |
| Volume and issue | 23 |
| Article # | 391 |
| DOI | 10.1186/s12909-023-04272-7 |
| ISSN | 1472-6920 |
| Distribution license | Creative Commons Attribution 4.0 International |
| Website | https://bmcmededuc.biomedcentral.com/articles/10.1186/s12909-023-04272-7 |
| Download | https://bmcmededuc.biomedcentral.com/counter/pdf/10.1186/s12909-023-04272-7.pdf (PDF) |
|
|
This article should be considered a work in progress and incomplete. Consider this article incomplete until this notice is removed. |
Abstract
Background: The error-proneness in the pre-analytical and post-analytical stages is higher than that in the analytical stage of the total laboratory testing process. However, pre-analytical and post-analytical quality management has not received enough attention in medical laboratory education and tests in clinical biochemistry courses.
Methods/approach: Clinical biochemistry teaching programs aim to improve students’ awareness of and ability to use quality management practices according to the International Organization for Standardization's ISO 15189 requirements. We designed a student-centered laboratory training program according to case-based learning that included four stages: establish an overall testing process based on the patient’s clinical indicators, clarify principles, improve operational skills, and review processes and continuous improvement opportunities. The program was implemented in our college during the winter semesters of 2019 and 2020. A total of 185 undergraduate students majoring in medical laboratory science participated in the program as a test group, and the other 172 students were set up as the control group and adopted the conventional method. The participants were asked to finish an online survey to evaluate the class at the end.
Results/outcomes: The test group had significantly better examination scores not only in experimental operational skills (89.27 ± 7.16 vs. 77.51 ± 4.72, p < 0.05 in 2019 grade, 90.31 ± 5.35 vs. 72.87 ± 8.41 in 2020 grade) but also in total examination (83.47 ± 6.16 vs. 68.90 ± 5.86 in 2019 grade, 82.42 ± 5.72 vs. 69.55 ± 7.54 in 2020 grade) than the control group. The results of the questionnaire survey revealed that the students in the test group better achieved classroom goals than those in the control group (all p < 0.05).
Conclusions: The new student-centered laboratory training program based on case-based learning in clinical biochemistry is an effective and acceptable strategy compared with the conventional training program.
Keywords: case-based learning, clinical biochemistry, laboratory training, quality management, student-centered
Introduction
Clinical biochemistry is a pivotal division of the modern medical laboratory. According to the International Federation of Clinical Chemistry (IFCC), clinical chemistry is responsible for applying chemical, molecular, and cellular strategies and techniques to better understand and assess human health and disease processes. It ultimately affects the process of treatment as well as the quality of medical outcomes. [1] It has been reported that the results of laboratory tests influence 70 percent of medical diagnoses, guide approximately 70 percent of clinical decisions, and facilitate the provision of optimal patient care. [2, 3]
Practical training plays a crucial role in clinical biochemistry curriculum. The goal of the course is to enable students to remember the test procedure and understand the underlying principles and medical significance, especially to ensure the accuracy of the test results. However, in traditional teaching, emphasis on quality control (QC) during the analytical process has received more attention, while neglecting elements of QC during the pre-analytical and post-analytical processes in the experimental courses of clinical biochemistry teaching. In fact, the error-proneness in the analytical process is lower than that in pre- and post-analytical processes of the total testing process (TTP). [4] Moreover, awareness of and ability to use quality management practices are much more important for students. The International Organization for Standardization's (ISO's) ISO 15189 Medical laboratories — Requirements for quality and competence was first published by the ISO's Clinical laboratory testing and in vitro diagnostic test systems technical committee (ISO/TC 212) in 2003. After several revisions, it has become an important international gold standard in medical laboratory proficiency, cultivating strong elements of laboratory quality management while addressing the processes and procedures that should be used throughout the TTP. [5, 6] As such, there is strong value in acclimating students to the concepts of quality management by applying the ISO 15189 standard to clinical biochemistry classwork.
Traditional training models such as lecture-based learning (LBL) have several features, including a teacher-centered tiered process, a focus on knowledge acquisition, and a final summative assessment at the end of courses. This is indeed the most cost-effective way to carry out theoretical education. [7] As such, several teaching modes are obviously superior to traditional teaching in the course of clinical biochemistry, such as traditional teaching combined with group discussion, peer debriefing approaches, and team learning. [8,9,10] However, small groups and case-based learning (CBL) are likely to dominate medical education. CBL is a learner-centered special type of problem-based learning (PBL) that guides students’ learning and exploration through cases. It has been elucidated that CBL can improve the performance and clinical skills of medical students [11]; help convey an understanding of key concepts [12]; improve clinical practice, problem-solving, case analysis, and the link between theory and practice [13,14,15]; and motivate students to learn more deeply [16], with better student satisfaction. [17] It is hypothesized that students who participate in CBL gain deeper and longer lasting knowledge than those who do not. [18] Compared with traditional methods, the application of practical knowledge (Objective Structure Clinic Examination, OSCE scores) through CBL is significantly improved. [19]
A limitation of this approach is that multiple faculty facilitators may be needed. However, during the COVID-19 pandemic, virtual teaching workshops emerged as an easy and straightforward way to implant a more interactive format into virtual case teaching for health professions [20]. That being said, there is yet to appear a proper teaching model that focuses on improving the entire quality management process dictated by ISO 15189 in clinical biochemistry courses.
Here, we designed a new student-centered training program based on CBL in the experimental teaching of a clinical biochemistry course, with the goal of improving the awareness of and ability to use quality management practices by students majoring in medical laboratory science.
Methods
Participants
A total of 357 undergraduate students majoring in medical laboratory science in 2019 and 2020 were randomly divided into two groups: a test group and a control group. Students participated in the program each semester. There were 92 recruited into the testing group in 2019 and 93 recruited into 2020 according to individual will. The number of male and female students was kept similar to exclude the influencing factors of gender on CBL. [13] The remaining students (87 in 2019, 85 in 2020) participated in the traditional program as a control group. Teachers with at least one year of CBL teaching experience were designated as the teachers of the test group, which enrolled 10 to 12 students per training classroom. All study participants completed basic medical courses related to the testing profession and had a certain ability to comprehensively analyze medical knowledge. Students from both groups were taught by the same teachers using the same syllabus and teaching materials. In this study, no significant differences were found between the study participants, such as the theoretical score of biochemistry and clinical disease synopsis course. The control group was given appropriate supplementary training after the examination to prevent perceived unfairness in their education experience. All the programs were approved by the education committee of our college.
Teaching strategies
A total of nine experiments were assigned. The primary subject was on biosafety and the use of biochemical instruments commonly used in clinical practice. The themes of the remaining eight classes involved specific experiments on clinical indicators of diabetes mellitus, liver cirrhosis, nephrotic syndrome, coronary atherosclerotic cardiopathy, pancreatitis, electrolyte disturbance, multiple myeloma, and hyperthyroidism. At the end of program, the lab examination was performed. Each experiment was conducted in three consecutive classes of 45 minutes. A similar learning environment was maintained for both groups, i.e., lab classrooms, lecture times, assessment methods.
Test group
The laboratory training adopted a new student-centered training program that was divided into four stages. First, students had access to the case (with the questions) at least two to four days before the class and were asked to answer several basic questions individually about the case before the class, e.g., what the diagnosis is based on, what the detection indicators are, and what the the indicators of a certain inspection procedures for pre-analysis, analysis, and post-analysis are. The answers of each minor group were then shared in the class, and the students tried to reach a consensus among the groups, with the teachers’ facilitation. This stage took approximately 30 minutes. Second, it took 30 minutes to learn principles, which was mainly an explanation of the current commonly used methods and principles. Third, it took 45 minutes to improve their lab skills, including the evaluation of lab conditions, assessment of equipment conditions, use of internal control, and sample processing according to the standard operating procedure (SOP). Fourth, results were analyzed by combining the ISO 15189 requirements with the teaching contents to improve the operations in 30 minutes. The main concern was the review and reporting of results. When abnormal or suspicious results occurred, the students were able to identify them. The teachers facilitated the entire process. If the results were not judged correctly, the teacher asked students to re-check the result until they met the re-inspection requirements, and the students analyzed whether the results could be issued. After that, the students were asked to conduct a quiz and an after-class survey.
Control group
The knowledge and theoretical outline of the clinical biochemistry course in the lectures was the same as that of the test group. Experimental teaching was implemented in a teacher-centered way. The teacher explained the principles, operation points, and medical significance, and then the students performed the experiment. A schematic diagram of the teaching mode between the two groups is shown in Figure 1.
|
Here is an example. Students became familiar with the clinical manifestations of a patient with recurrent systemic edema (finally diagnosed as nephrotic syndrome) two weeks before the class on that same topic. When the nephrotic syndrome was diagnosed, urinalysis, blood counts and coagulation panel, renal function and electrolytes, liver panel, and glucose tests were required. Students were asked to report why and how the test procedure for “creatine and urea” in renal function was determined. Finally, students were then asked to discuss the examination process and medical significance, and analyze various factors that may affect the test result, including pre-pre-analysis (i.e., test selection, test ordering, patient/specimen identification), pre-analysis (i.e., specimen collection, transportation, specimen processing, specimen preparation), analytic, post-analysis (i.e., report review, result reporting), and post-post-analysis (i.e., result interpretation) in accordance with the requirements of ISO 15189. [21] Then students made an operation plan according to the inspection process of the project, and the teachers evaluated and determined the testing procedure.
Outcome evaluation
Assessment for laboratory operation
To evaluate students’ students’ awareness of and ability to use quality management practices in TTP and other laboratory processes, evaluation indicators were designed as shown in Table 1.
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
References
Notes
This presentation is faithful to the original, with only a few minor changes to presentation, spelling, and grammar. In some cases important information was missing from the references, and that information was added.