Difference between revisions of "Template:Article of the week"
Shawndouglas (talk | contribs) (Updated article of the week text.) |
Shawndouglas (talk | contribs) (Added last week's article of the week) |
||
| Line 1: | Line 1: | ||
<div style="float: left; margin: 0.5em 0.9em 0.4em 0em;">[[File:Fig2 | <div style="float: left; margin: 0.5em 0.9em 0.4em 0em;">[[File:Fig2 Pillai FrontBioengBiotech2022 10.jpg|120px]]</div> | ||
'''"[[Journal: | '''"[[Journal:Practical considerations for laboratories: Implementing a holistic quality management system|Practical considerations for laboratories: Implementing a holistic quality management system]]"''' | ||
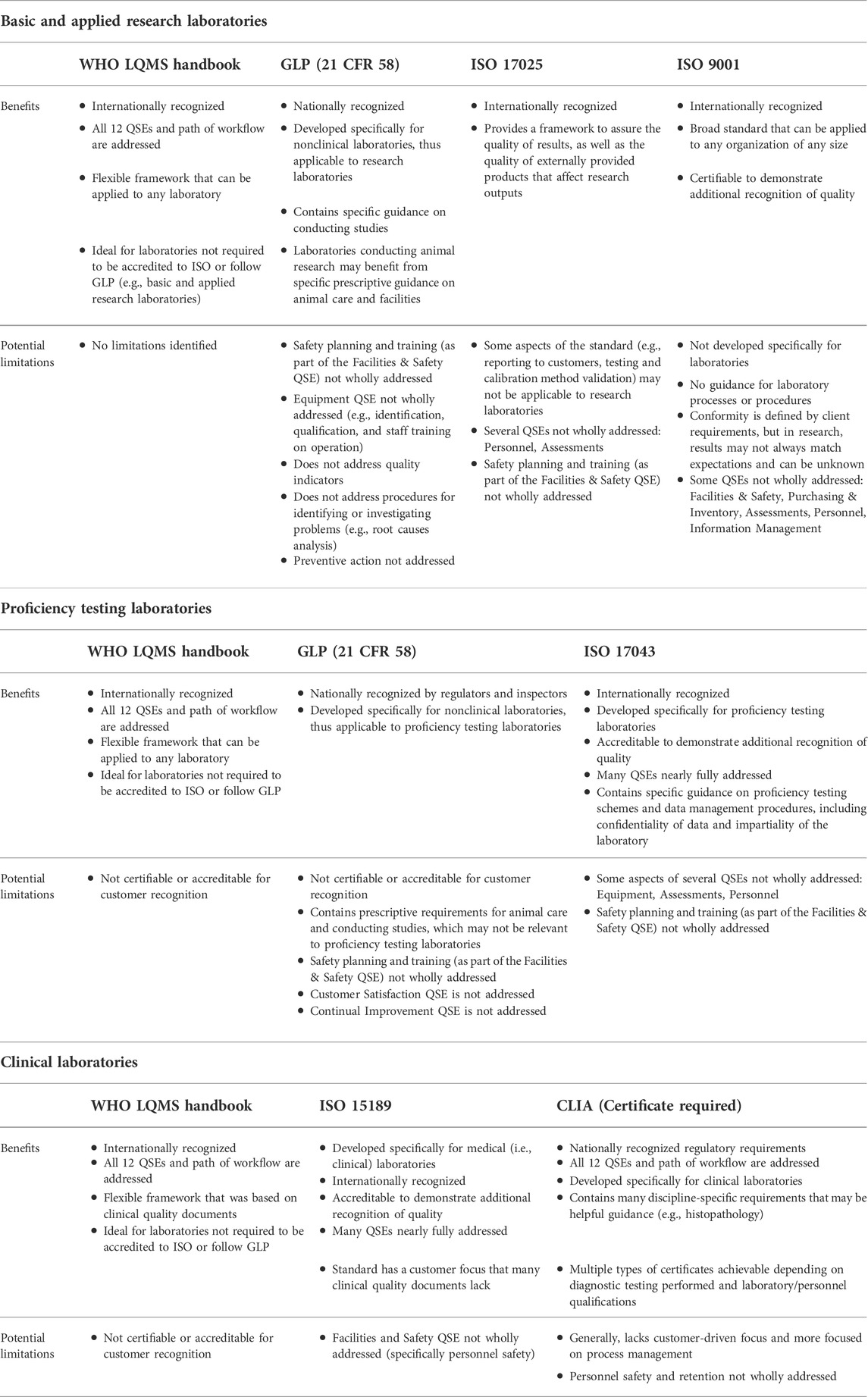
A [[quality management system]] (QMS) is an essential element for the effective operation of [[research]], clinical, testing, or production/manufacturing [[Laboratory|laboratories]]. As technology continues to rapidly advance and new challenges arise, laboratories worldwide have responded with innovation and process changes to meet the continued demand. It is critical for laboratories to maintain a robust QMS that accommodates laboratory activities (e.g., basic and applied research; regulatory, clinical, or proficiency testing), records management, and a path for [[Continual improvement process|continuous improvement]] to ensure that results and data are reliable, accurate, timely, and reproducible. A robust, suitable QMS provides a framework to address gaps and risks throughout the laboratory's [[workflow]] that could potentially lead to a critical error, thus compromising the integrity and credibility of the institution. While there are many QMS frameworks (e.g., a model such as a consensus standard, guideline, or regulation) that may apply to laboratories, ensuring that the appropriate framework is adopted based on the type of work performed and that key implementation steps are taken is important for the long-term success of the QMS and for the advancement of science ... ('''[[Journal:Practical considerations for laboratories: Implementing a holistic quality management system|Full article...]]''')<br /> | |||
''Recently featured'': | ''Recently featured'': | ||
{{flowlist | | {{flowlist | | ||
* [[Journal:Precision nutrition: Maintaining scientific integrity while realizing market potential|Precision nutrition: Maintaining scientific integrity while realizing market potential]] | |||
* [[Journal:Construction of control charts to help in the stability and reliability of results in an accredited water quality control laboratory|Construction of control charts to help in the stability and reliability of results in an accredited water quality control laboratory]] | * [[Journal:Construction of control charts to help in the stability and reliability of results in an accredited water quality control laboratory|Construction of control charts to help in the stability and reliability of results in an accredited water quality control laboratory]] | ||
* [[Journal:Application of informatics in cancer research and clinical practice: Opportunities and challenges|Application of informatics in cancer research and clinical practice: Opportunities and challenges]] | * [[Journal:Application of informatics in cancer research and clinical practice: Opportunities and challenges|Application of informatics in cancer research and clinical practice: Opportunities and challenges]] | ||
}} | }} | ||
Revision as of 15:18, 29 May 2023
"Practical considerations for laboratories: Implementing a holistic quality management system"
A quality management system (QMS) is an essential element for the effective operation of research, clinical, testing, or production/manufacturing laboratories. As technology continues to rapidly advance and new challenges arise, laboratories worldwide have responded with innovation and process changes to meet the continued demand. It is critical for laboratories to maintain a robust QMS that accommodates laboratory activities (e.g., basic and applied research; regulatory, clinical, or proficiency testing), records management, and a path for continuous improvement to ensure that results and data are reliable, accurate, timely, and reproducible. A robust, suitable QMS provides a framework to address gaps and risks throughout the laboratory's workflow that could potentially lead to a critical error, thus compromising the integrity and credibility of the institution. While there are many QMS frameworks (e.g., a model such as a consensus standard, guideline, or regulation) that may apply to laboratories, ensuring that the appropriate framework is adopted based on the type of work performed and that key implementation steps are taken is important for the long-term success of the QMS and for the advancement of science ... (Full article...)
Recently featured:
- Precision nutrition: Maintaining scientific integrity while realizing market potential
- Construction of control charts to help in the stability and reliability of results in an accredited water quality control laboratory
- Application of informatics in cancer research and clinical practice: Opportunities and challenges