Difference between revisions of "Journal:Integrative diagnostics: The time is now—a report from the International Society for Strategic Studies in Radiology"
Shawndouglas (talk | contribs) (Saving and adding more) |
Shawndouglas (talk | contribs) (Saving and adding more) |
||
| Line 47: | Line 47: | ||
===What is ID?=== | ===What is ID?=== | ||
More than seven billion diagnostic examinations are performed each year in the United States, influencing 70% of health care decisions. [4] Although [[Medical test|diagnostic tests]] differ in personnel, infrastructure, and technology, they have a shared commonality: providing data for clinical diagnosis. [5] ID has been proposed to better manage, organize, and present diagnostic data and bridge intellectual silos. ID represents a convergence of imaging, pathology, and clinical laboratory medicine, plus advanced levels of IT. [6] In this framework, integrated (versus isolated) practices plus [[Clinical decision support system|clinical decision support (CDS) tools]] drive appropriate care. Data from the entire diagnostic arsenal are aggregated to enhance insights, and EHRs present information in a consumable way to facilitate collaborative decision making and accurate clinical diagnosis. ID uses [[medical informatics]] (in which data are data, regardless of their nature or source) to organize and analyze vast, disparate diagnostic data sets to achieve timely and accurate diagnosis, precise therapeutics, accurate assessment of prognosis, and maintenance of population health. [7] | More than seven billion diagnostic examinations are performed each year in the United States, influencing 70% of health care decisions. [4] Although [[Medical test|diagnostic tests]] differ in personnel, infrastructure, and technology, they have a shared commonality: providing data for clinical diagnosis. [5] ID has been proposed to better manage, organize, and present diagnostic data and bridge intellectual silos. ID represents a convergence of imaging, pathology, and clinical laboratory medicine, plus advanced levels of IT. [6] In this framework, integrated (versus isolated) practices plus [[Clinical decision support system|clinical decision support (CDS) tools]] drive appropriate care. Data from the entire diagnostic arsenal are aggregated to enhance insights, and EHRs present information in a consumable way to facilitate collaborative decision making and accurate clinical diagnosis. ID uses [[medical informatics]] (in which data are data, regardless of their nature or source) to organize and analyze vast, disparate diagnostic data sets to achieve timely and accurate diagnosis, precise therapeutics, accurate assessment of prognosis, and maintenance of population health. [7] | ||
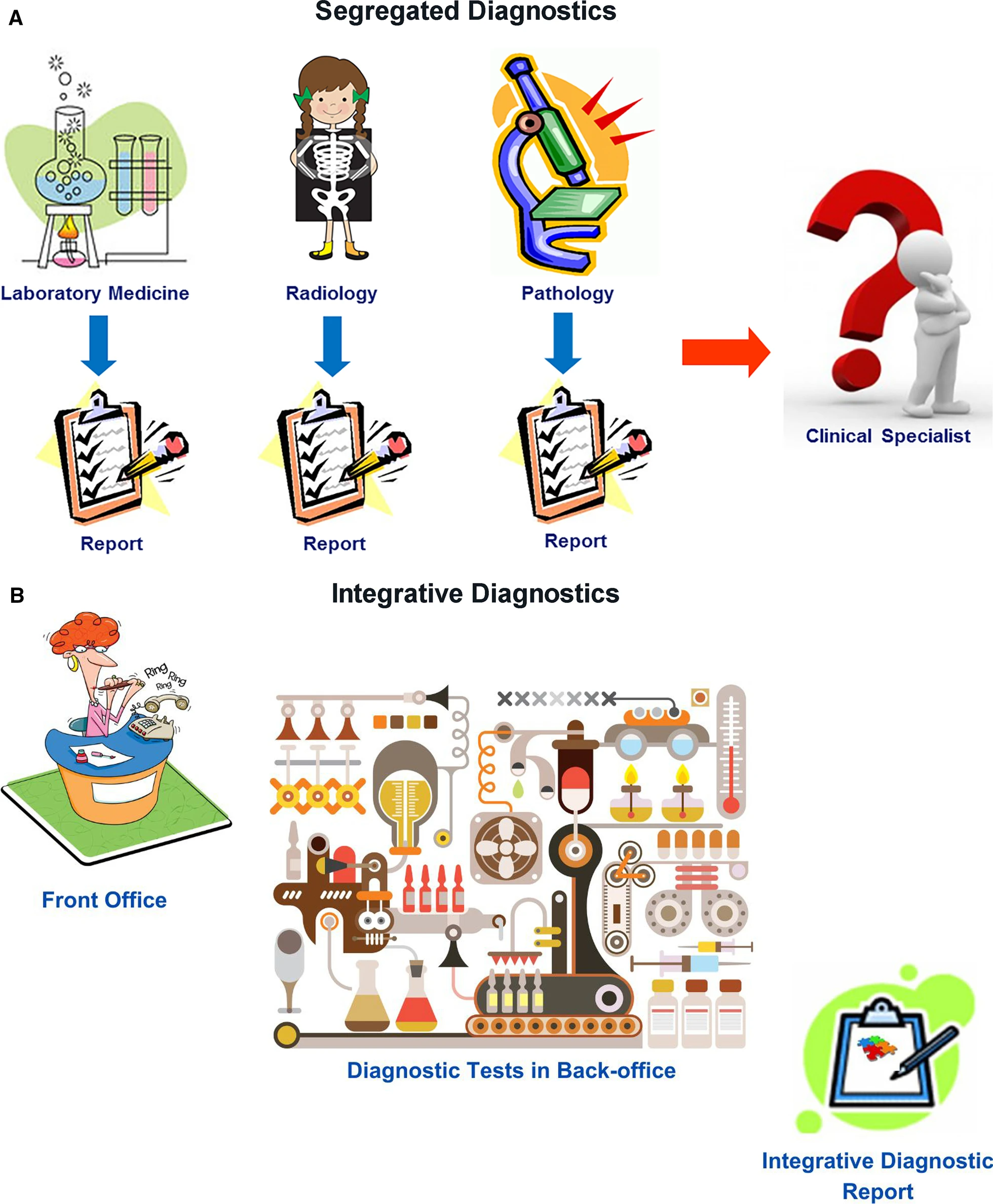
Radiology, [[Clinical laboratory|clinical laboratories]], and pathology departments, which perform the preponderance of diagnostic tests, currently play a central role in medical diagnostics. However, our disciplines have not worked as an integrated unit. Rather, we are islands of vast data and extraordinary intradisciplinary expertise separated from one another and from our clinical colleagues by [[Informatics (academic field)|informatics]], physical, and specialty barriers. We have not integrated our data or communicated them in a coordinated fashion to our clinical colleagues, instead expecting clinicians to integrate and interpret these data themselves. Although of immense potential value, our petabytes of data are increasingly overwhelming providers and systems as we “throw our work over the fence” and hope that someone figures out what it all means (Fig. 1A). It is no longer possible for individual health care providers to perform this complex task. ID offers a helping hand. | |||
[[File:FIG1 Beauchamp InsightsImaging2022 14.png|600px]] | |||
{{clear}} | |||
{| | |||
| style="vertical-align:top;" | | |||
{| border="0" cellpadding="5" cellspacing="0" width="600px" | |||
|- | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |<blockquote>'''Figure 1.''' Diagram describing (A') segregated diagnostics versus (B) integrative diagnostics.</blockquote> | |||
|- | |||
|} | |||
|} | |||
In ID, radiologists, pathologists, and other diagnosticians work as teams with shared access to continuously updated patient data, from which experts and CDS tools extract relevant clinical information and formulate dynamic differential diagnosis and management pathways (Fig. 1B). Given our in-depth knowledge of our test data, understanding of the pathobiochemical and physiological basis of our diagnostic findings, technological skills, and strong informatics resources and expertise, the practices of radiology and pathology should strive for leadership roles in the ID environment. | |||
Predictive analytic tools based on aggregated clinical data can streamline care pathways so that appropriate diagnostic tests (including those performed by radiology, laboratory medicine, and pathology) are expedited on the basis of reason for referral, even in advance of a patient’s visit with a provider. This requires real-time data entry from all sources, continual analytics, and timely interactive communication among [[Laboratory|laboratories]], providers, and patients. Triaging patients in this manner could streamline and more appropriately prioritize health care access. For example, by identifying patients who need to be seen sooner, a decrease in wait times for specialists would provide reassurance to patients earlier in their care journey and prevent them from turning to high-cost settings such as the emergency department for care. ID could direct patients to the correct therapy sooner, modify treatment when appropriate, and terminate it when not effective, ultimately decreasing morbidity, improving outcomes, and avoiding unnecessary cost. Earlier access and more appropriate care are increasingly rewarded in value-based care payment arrangements. Additionally, ID could assess information that affects both individual patient well-being and population health, including identification of emerging infections, antibiotic resistance, exposure to toxic substances, and chemical or biologic threats. | |||
Despite a clear need and sound theoretical reasons for expanding the role of radiologists and pathologists in ID, real-world efforts remain meager. Our purpose is to stimulate more ID activity in our specialties by presenting the rationale for such efforts, highlighting successful ID programs that might be emulated at other sites, and recommending specific endeavors that are feasible now and should be prioritized in our departments and institutions. | |||
===The ID process=== | |||
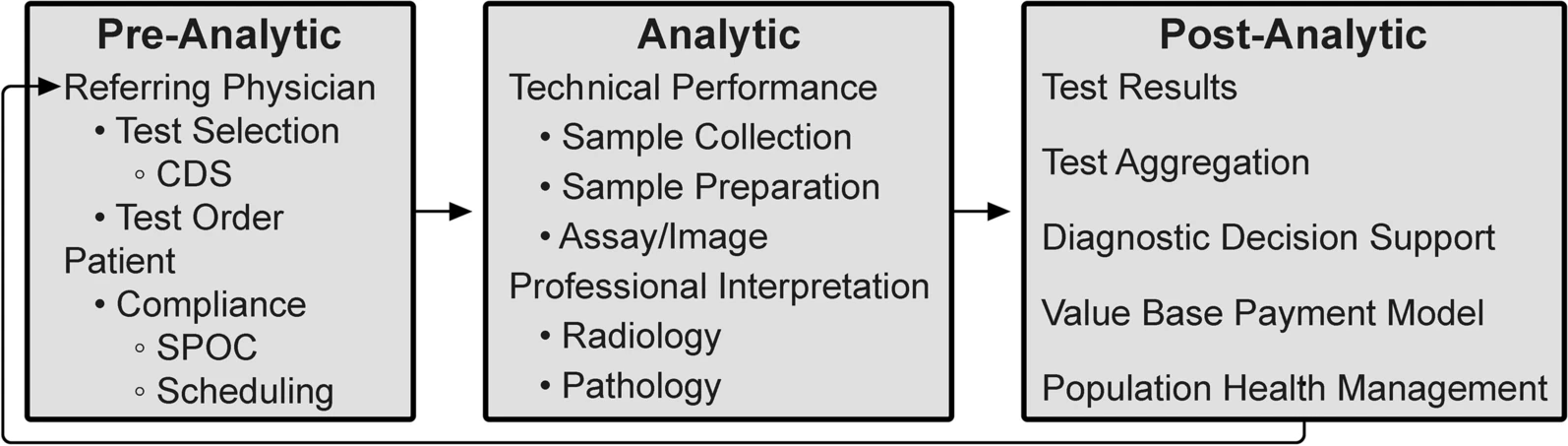
As outlined by the Institute of Medicine Committee on Diagnostic Error in Health Care, the diagnostic examination process is divided into three phases: pre-analytic, analytic, and post-analytic. [7, 8] (Fig. 2) | |||
[[File:Fig2 Beauchamp InsightsImaging2022 14.png|600px]] | |||
{{clear}} | |||
{| | |||
| style="vertical-align:top;" | | |||
{| border="0" cellpadding="5" cellspacing="0" width="600px" | |||
|- | |||
| style="background-color:white; padding-left:10px; padding-right:10px;" |<blockquote>'''Figure 2.''' Integrative diagnostic workflow phases. CDS = clinical decision support; SPOC = single point of contact.</blockquote> | |||
|- | |||
|} | |||
|} | |||
The analytic phase is the least susceptible to errors because of attention to technical performance and procedural standards, rigorous internal management and external quality assessment, and precise quantitative measurements. In contrast, our relative inattention to the pre- and post-analytic phases now warrants modification. In laboratory medicine, the analytic phase accounts for approximately 25% of total effort and [[workflow]], the pre-analytic phase for 57%, and the post-analytic phase for 17%. [9] A disease process that requires inputs from multiple diagnostic disciplines is typically interrogated in a stepwise and discontinuous way. Although this is sometimes unavoidable (including subsequent testing whose utility only becomes apparent on the basis of preceding tests), the fragmented, sequential nature of the diagnostic process can cause treatment delays with negative impacts on outcomes. [10] ID can accelerate medical diagnosis, transforming this discontinuous, slow, and fragmented approach into a highly coordinated process with faster information flow through these test phases. | |||
In the pre-examination phase, the referring provider is responsible for performing and/or requesting the most appropriate examinations. In an estimated 10 to 15% of cases, the referring provider needs, and would value, assistance with these decisions. Inappropriate laboratory testing involves both under- and overutilization and occurs in 20 to 30% of cases. [11] Extra support may be needed for primary care providers, who see a wide variety of patients and diseases. [12] Modern health care informatics can help providers request the most appropriate examinations by integrating CDS tools into clinical workflow. [13] For example, use of a real-time radiology appropriateness CDS application decreased inappropriate utilization of brain and spine MRI and sinus CT [14], and computer-generated reminders to clinicians in Kenya improved CD4 laboratory monitoring of patients with HIV infection. [15] CDS tools should be available on platforms that integrate seamlessly into providers’ workflow without adding unnecessary steps, and the appropriate use criteria underpinning these tools must be evidence-based. Feedback to providers must be supportive and advance learning, rather than being punitive. Additionally, CDS systems should be adapted to local circumstances, including local patient demographics and resources (such as the availability of diagnostic test equipment and the competencies of diagnosticians). CDS systems must also be capable of incorporating all relevant patient data. Moreover, CDS should be “integrated,” reflecting not only the appropriateness of a single diagnostic discipline but also the benefit of combinations of tests across disciplines. | |||
Although referring physicians are responsible for maximizing the likelihood that patients will get needed examinations, approximately 20% of requested examinations in the United States are never performed. [16] Well-designed health care systems using contemporary, web-connected logistic support tools can improve this by coordinating examination times across disciplines at sites that match patients’ circumstances and preferences to local health care resources. [[Point-of-care testing|Point-of-care (POC) testing]] and service increases patient test completion, satisfaction, and clinical outcomes, although it presents efficiency and [[quality control]] (QC) challenges. POC laboratory testing has improved clinical outcomes in influenza and pneumonia, HIV infection, heart attack, and strep throat. [17] In Berlin, the use of mobile stroke units with CT scans and POC laboratory tests resulted in decreased time to treatment and lower global disability at three-month follow-up. [18] | |||
The analytic phase of the diagnostic process centers on the performance of each specific examination, which are currently relatively independent events. Most [[picture archiving and communication system]]s (PACS) and [[radiology information system]]s (RIS) are separate from [[laboratory information system]]s (LIS), resulting in each pathologist and radiologist interpreting their own studies without easy access to the others’ results. Bridging this disconnect should be a radiology and pathology IT priority. Diagnostic accuracy and management recommendations are improved when examinations are tailored and interpreted using knowledge from previous tests. Modern informatics, through optimizing the EHR, should make the complete medical record available to every examiner at the time of an examination, along with appropriate management guidelines. Each study’s unique information should be intelligently and intuitively encapsulated in each sequential result. For example, POC CDS for management of incidental lung nodules improved adherence to nationally recommended guidelines for follow-up. [19] | |||
The post-analytic phase of diagnostics initially focuses on the application of test results to the individual patient’s diagnosis and care plan. The aggregation and diagnostic inferences from all of a patient’s examinations leads to the most accurate and specific diagnosis. Examination results should be promptly and intuitively incorporated into the EHR and made easily available to health care providers and the patient. Until the past decade, these medical data were analyzed by a single or small number of providers, primarily using heuristics. Although an invaluable human thought process, heuristics takes shortcuts in reasoning, may not use all available data, and has well-known sources of error, including cognitive, selective, and availability biases. [20] Furthermore, heuristics suffers reduced accuracy and efficiency with increasing volumes and diversity of data types and greater task complexity. | |||
Deficiencies in current practice and EHRs extend from the most basic error—missing data—to data overload, as with radiopathogenomics. For example, in a Veterans Affairs setting, 30% of providers reported encountering at least one patient with a missed test result over the previous two weeks that caused a delay in diagnosis or treatment. [21] Tumor boards, with their extensive clinical, imaging, laboratory, and [[Anatomical pathology|anatomic pathology]] content, may represent the epitome of data overload. No single human can master all the information of even one patient in any reasonable period of time, which was the topic of an RSNA/American Association of Physicists in Medicine symposium on ID in 2019. [22] The current explosion of remote health monitoring tools and diagnostic tests with exponentially larger data units can easily overwhelm a single astute physician. It is estimated that every patient generates 80 MB of data each year, and the volume of health care data is predicted to increase faster than any other business sector. [23, 24] ID can bring more human and computational resources to bear on these essentially raw data to yield useful information to diagnose and treat individual patient problems as well as address population disease and health management. [25] | |||
An intelligent informatics infrastructure leveraging all these individual and population data can greatly augment the traditional human analysis. Integrated structured reports with discrete data are critical for data aggregation, which, in conjunction with outcome data, allows the development and optimization of front-end CDS systems. CDS tools, incorporating [[artificial intelligence]] (AI) and [[machine learning]] (ML) methodology, can provide referring providers with real-time probabilistic differential diagnoses for individual patients and enable the development of management paradigms for specific diseases and large populations. [26] Unfortunately, many CDS systems are monodisciplinary, prematurely obsolete, and incompatible with efficient clinical workflow. Modern health care informatics must develop fully integrated CDS tools that are multidisciplinary, continuously updated, and adapted to the local situation. These new management paradigms can close the loop from the post-analytic back to the pre-analytic phase by suggesting the most appropriate examinations for the referring physician’s next patient with a similar problem set. Furthermore, this information can help health care systems identify their most burdensome and needy patients for proactive health care management. | |||
In the United States, the 21st Century Cures Act emphasizes the patient’s role in the post-analytic phase. The program rule on interoperability, information blocking, and Office of National Health Coordinator (ONHC) health IT certification, which implements this act, requires that health care providers give patients access without charge to all the health information in their EHR “without delay.” [27] This legislative directive, predicated on evidence that optimal diagnosis and treatment are enhanced by the convenient availability of patients’ medical records to their physicians, will further drive the aggregation of medical information, regardless of initial source or current repository. This initiative should better enable all physicians involved in a patient’s care to have immediate access to all of that patient’s health care information. Although providing invaluable information to providers, including radiologists and pathologists, the technical and legal demands of this act on provider practices, health systems, and IT vendors will be significant. The requirement that all components of the EHR be promptly provided to the patient, including all laboratory, anatomic pathology, and radiology reports, presents a communication challenge and an opportunity for ID. We will have to modify our reports for patients’ consumption through health care portals, offering the opportunity to integrate and summarize test results for patients and referring physicians. | |||
Revision as of 21:49, 3 April 2023
| Full article title | Integrative diagnostics: The time is now—a report from the International Society for Strategic Studies in Radiology |
|---|---|
| Journal | Insights into Imaging |
| Author(s) | Beauchamp, Norman J.; Bryan R. Nick; Bui, Marilyn M.; Krestin, Gabriel P.; McGinty, Geraldine B.; Meltzer, Carolyn C.; Neumaier, Michael |
| Author affiliation(s) | Michigan State University, University of Pennsylvania, Moffitt Cancer Center and Research Institute, University Medical Center Rotterdam, Weill Cornell Medicine, University of Southern California, University of Heidelberg |
| Primary contact | Email: robert dot bryan at pennmedicine dot upenn dot edu |
| Year published | 2022 |
| Volume and issue | 14 |
| Article # | 54 |
| DOI | 10.1186/s13244-023-01379-9 |
| ISSN | 1869-4101 |
| Distribution license | Creative Commons Attribution 4.0 International |
| Website | https://insightsimaging.springeropen.com/articles/10.1186/s13244-023-01379-9 |
| Download | https://insightsimaging.springeropen.com/counter/pdf/10.1186/s13244-023-01379-9.pdf (PDF) |
|
|
This article should be considered a work in progress and incomplete. Consider this article incomplete until this notice is removed. |
Abstract
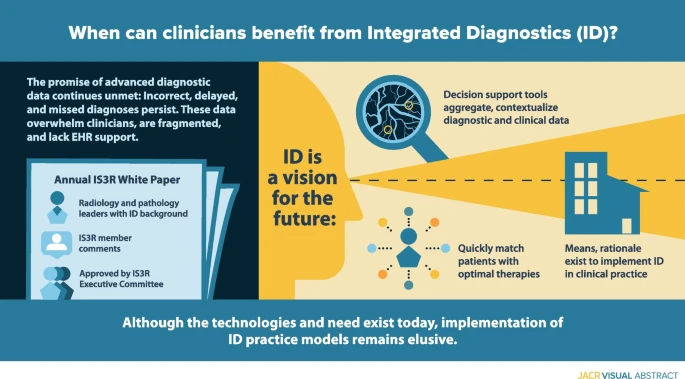
Enormous recent progress in diagnostic testing can enable more accurate diagnosis and improved clinical outcomes. Yet these tests are increasingly challenging and frustrating; the volume and diversity of results may overwhelm the diagnostic acumen of even the most dedicated and experienced clinician. Because they are gathered and processed within the “silo” of each diagnostic discipline, diagnostic data are fragmented, and the electronic health record (EHR) does little to synthesize new and existing data into usable information. Therefore, despite great promise, diagnoses may still be incorrect, delayed, or never made. Integrative diagnostics represents a vision for the future, wherein diagnostic data, together with clinical data from the EHR, are aggregated and contextualized by informatics tools to direct clinical action. Integrative diagnostics has the potential to identify correct therapies more quickly, modify treatment when appropriate, and terminate treatment when not effective, ultimately decreasing morbidity, improving outcomes, and avoiding unnecessary costs. Radiology, laboratory medicine, and pathology already play major roles in medical diagnostics. Our specialties can increase the value of our examinations by taking a holistic approach to their selection, interpretation, and application to the patient’s care pathway. We have the means and rationale to incorporate integrative diagnostics into our specialties and guide its implementation in clinical practice.
The following overall points can be made about this research:
- Although an overall boon to clinical diagnosis, increasingly voluminous, diverse, and fragmented diagnostic data can overwhelm physicians and frustrate patients.
- Data are gathered and processed within “silos” of the diagnostic disciplines, including radiology and pathology. The EHR does little to intelligently organize and synthesize these disparate data to facilitate diagnosis.
- Integrative diagnostics envisions a process in which data from the entire arsenal of in vivo and in vitro diagnostics, together with clinical data from the EHR, are aggregated and contextualized to enhance diagnosis and direct clinical action.
Keywords: diagnostics, integrative diagnostics, radiology, pathology, informatics
Background
Despite digital imaging, widespread adoption of electronic health records (EHRs), and advances in precision medicine tools, diagnosis often remains a fragmented and frustrating process for clinicians and patients. Data are still gathered and presented asynchronously, and EHRs do little to organize and synthesize information. Although team practice, such as tumor boards, is increasing, routine physician interaction is limited by clinical workflow, high volumes, and information technology (IT) boundaries. Despite an abundance of relevant diagnostic data, diagnoses may be incorrect, delayed, or never made. Allegations of diagnostic errors account for 28% of malpractice cases in the United States. [1] Experts estimate a diagnostic error rate of 10% to 15%, with 40,000–80,000 preventable deaths each year. [2, 3] As physicians and diagnosticians, it is our responsibility to minimize these errors. Integrative diagnostics (ID) has been proposed as one means to reduce diagnostic errors.
Method
This paper is designed to address ID from the perspectives of radiology and our sister diagnostic specialty, pathology. The paper was developed in response to a request for proposal from the International Society of Strategic Studies in Radiology (IS3R) to its members for an annual white paper to foster its mission “to actively shape the future of medical imaging and image-guided therapies by leveraging the knowledge and influence of world leaders in these disciplines and related industries.” Proposals from self-organized writing groups were reviewed by the IS3R Publications Committee, with the final selection approved for drafting by the IS3R Executive Committee. Our writing group was designed to include departmental and institutional leaders in radiology and pathology who have interest and experience in ID. After preliminary approval by the Publications Committee, the draft paper was posted to the entire IS3R membership for comments, which were incorporated into this final document. This paper was approved for internal dissemination and publication by the IS3R Executive Committee.
What is ID?
More than seven billion diagnostic examinations are performed each year in the United States, influencing 70% of health care decisions. [4] Although diagnostic tests differ in personnel, infrastructure, and technology, they have a shared commonality: providing data for clinical diagnosis. [5] ID has been proposed to better manage, organize, and present diagnostic data and bridge intellectual silos. ID represents a convergence of imaging, pathology, and clinical laboratory medicine, plus advanced levels of IT. [6] In this framework, integrated (versus isolated) practices plus clinical decision support (CDS) tools drive appropriate care. Data from the entire diagnostic arsenal are aggregated to enhance insights, and EHRs present information in a consumable way to facilitate collaborative decision making and accurate clinical diagnosis. ID uses medical informatics (in which data are data, regardless of their nature or source) to organize and analyze vast, disparate diagnostic data sets to achieve timely and accurate diagnosis, precise therapeutics, accurate assessment of prognosis, and maintenance of population health. [7]
Radiology, clinical laboratories, and pathology departments, which perform the preponderance of diagnostic tests, currently play a central role in medical diagnostics. However, our disciplines have not worked as an integrated unit. Rather, we are islands of vast data and extraordinary intradisciplinary expertise separated from one another and from our clinical colleagues by informatics, physical, and specialty barriers. We have not integrated our data or communicated them in a coordinated fashion to our clinical colleagues, instead expecting clinicians to integrate and interpret these data themselves. Although of immense potential value, our petabytes of data are increasingly overwhelming providers and systems as we “throw our work over the fence” and hope that someone figures out what it all means (Fig. 1A). It is no longer possible for individual health care providers to perform this complex task. ID offers a helping hand.
|
In ID, radiologists, pathologists, and other diagnosticians work as teams with shared access to continuously updated patient data, from which experts and CDS tools extract relevant clinical information and formulate dynamic differential diagnosis and management pathways (Fig. 1B). Given our in-depth knowledge of our test data, understanding of the pathobiochemical and physiological basis of our diagnostic findings, technological skills, and strong informatics resources and expertise, the practices of radiology and pathology should strive for leadership roles in the ID environment.
Predictive analytic tools based on aggregated clinical data can streamline care pathways so that appropriate diagnostic tests (including those performed by radiology, laboratory medicine, and pathology) are expedited on the basis of reason for referral, even in advance of a patient’s visit with a provider. This requires real-time data entry from all sources, continual analytics, and timely interactive communication among laboratories, providers, and patients. Triaging patients in this manner could streamline and more appropriately prioritize health care access. For example, by identifying patients who need to be seen sooner, a decrease in wait times for specialists would provide reassurance to patients earlier in their care journey and prevent them from turning to high-cost settings such as the emergency department for care. ID could direct patients to the correct therapy sooner, modify treatment when appropriate, and terminate it when not effective, ultimately decreasing morbidity, improving outcomes, and avoiding unnecessary cost. Earlier access and more appropriate care are increasingly rewarded in value-based care payment arrangements. Additionally, ID could assess information that affects both individual patient well-being and population health, including identification of emerging infections, antibiotic resistance, exposure to toxic substances, and chemical or biologic threats.
Despite a clear need and sound theoretical reasons for expanding the role of radiologists and pathologists in ID, real-world efforts remain meager. Our purpose is to stimulate more ID activity in our specialties by presenting the rationale for such efforts, highlighting successful ID programs that might be emulated at other sites, and recommending specific endeavors that are feasible now and should be prioritized in our departments and institutions.
The ID process
As outlined by the Institute of Medicine Committee on Diagnostic Error in Health Care, the diagnostic examination process is divided into three phases: pre-analytic, analytic, and post-analytic. [7, 8] (Fig. 2)
|
The analytic phase is the least susceptible to errors because of attention to technical performance and procedural standards, rigorous internal management and external quality assessment, and precise quantitative measurements. In contrast, our relative inattention to the pre- and post-analytic phases now warrants modification. In laboratory medicine, the analytic phase accounts for approximately 25% of total effort and workflow, the pre-analytic phase for 57%, and the post-analytic phase for 17%. [9] A disease process that requires inputs from multiple diagnostic disciplines is typically interrogated in a stepwise and discontinuous way. Although this is sometimes unavoidable (including subsequent testing whose utility only becomes apparent on the basis of preceding tests), the fragmented, sequential nature of the diagnostic process can cause treatment delays with negative impacts on outcomes. [10] ID can accelerate medical diagnosis, transforming this discontinuous, slow, and fragmented approach into a highly coordinated process with faster information flow through these test phases.
In the pre-examination phase, the referring provider is responsible for performing and/or requesting the most appropriate examinations. In an estimated 10 to 15% of cases, the referring provider needs, and would value, assistance with these decisions. Inappropriate laboratory testing involves both under- and overutilization and occurs in 20 to 30% of cases. [11] Extra support may be needed for primary care providers, who see a wide variety of patients and diseases. [12] Modern health care informatics can help providers request the most appropriate examinations by integrating CDS tools into clinical workflow. [13] For example, use of a real-time radiology appropriateness CDS application decreased inappropriate utilization of brain and spine MRI and sinus CT [14], and computer-generated reminders to clinicians in Kenya improved CD4 laboratory monitoring of patients with HIV infection. [15] CDS tools should be available on platforms that integrate seamlessly into providers’ workflow without adding unnecessary steps, and the appropriate use criteria underpinning these tools must be evidence-based. Feedback to providers must be supportive and advance learning, rather than being punitive. Additionally, CDS systems should be adapted to local circumstances, including local patient demographics and resources (such as the availability of diagnostic test equipment and the competencies of diagnosticians). CDS systems must also be capable of incorporating all relevant patient data. Moreover, CDS should be “integrated,” reflecting not only the appropriateness of a single diagnostic discipline but also the benefit of combinations of tests across disciplines.
Although referring physicians are responsible for maximizing the likelihood that patients will get needed examinations, approximately 20% of requested examinations in the United States are never performed. [16] Well-designed health care systems using contemporary, web-connected logistic support tools can improve this by coordinating examination times across disciplines at sites that match patients’ circumstances and preferences to local health care resources. Point-of-care (POC) testing and service increases patient test completion, satisfaction, and clinical outcomes, although it presents efficiency and quality control (QC) challenges. POC laboratory testing has improved clinical outcomes in influenza and pneumonia, HIV infection, heart attack, and strep throat. [17] In Berlin, the use of mobile stroke units with CT scans and POC laboratory tests resulted in decreased time to treatment and lower global disability at three-month follow-up. [18]
The analytic phase of the diagnostic process centers on the performance of each specific examination, which are currently relatively independent events. Most picture archiving and communication systems (PACS) and radiology information systems (RIS) are separate from laboratory information systems (LIS), resulting in each pathologist and radiologist interpreting their own studies without easy access to the others’ results. Bridging this disconnect should be a radiology and pathology IT priority. Diagnostic accuracy and management recommendations are improved when examinations are tailored and interpreted using knowledge from previous tests. Modern informatics, through optimizing the EHR, should make the complete medical record available to every examiner at the time of an examination, along with appropriate management guidelines. Each study’s unique information should be intelligently and intuitively encapsulated in each sequential result. For example, POC CDS for management of incidental lung nodules improved adherence to nationally recommended guidelines for follow-up. [19]
The post-analytic phase of diagnostics initially focuses on the application of test results to the individual patient’s diagnosis and care plan. The aggregation and diagnostic inferences from all of a patient’s examinations leads to the most accurate and specific diagnosis. Examination results should be promptly and intuitively incorporated into the EHR and made easily available to health care providers and the patient. Until the past decade, these medical data were analyzed by a single or small number of providers, primarily using heuristics. Although an invaluable human thought process, heuristics takes shortcuts in reasoning, may not use all available data, and has well-known sources of error, including cognitive, selective, and availability biases. [20] Furthermore, heuristics suffers reduced accuracy and efficiency with increasing volumes and diversity of data types and greater task complexity.
Deficiencies in current practice and EHRs extend from the most basic error—missing data—to data overload, as with radiopathogenomics. For example, in a Veterans Affairs setting, 30% of providers reported encountering at least one patient with a missed test result over the previous two weeks that caused a delay in diagnosis or treatment. [21] Tumor boards, with their extensive clinical, imaging, laboratory, and anatomic pathology content, may represent the epitome of data overload. No single human can master all the information of even one patient in any reasonable period of time, which was the topic of an RSNA/American Association of Physicists in Medicine symposium on ID in 2019. [22] The current explosion of remote health monitoring tools and diagnostic tests with exponentially larger data units can easily overwhelm a single astute physician. It is estimated that every patient generates 80 MB of data each year, and the volume of health care data is predicted to increase faster than any other business sector. [23, 24] ID can bring more human and computational resources to bear on these essentially raw data to yield useful information to diagnose and treat individual patient problems as well as address population disease and health management. [25]
An intelligent informatics infrastructure leveraging all these individual and population data can greatly augment the traditional human analysis. Integrated structured reports with discrete data are critical for data aggregation, which, in conjunction with outcome data, allows the development and optimization of front-end CDS systems. CDS tools, incorporating artificial intelligence (AI) and machine learning (ML) methodology, can provide referring providers with real-time probabilistic differential diagnoses for individual patients and enable the development of management paradigms for specific diseases and large populations. [26] Unfortunately, many CDS systems are monodisciplinary, prematurely obsolete, and incompatible with efficient clinical workflow. Modern health care informatics must develop fully integrated CDS tools that are multidisciplinary, continuously updated, and adapted to the local situation. These new management paradigms can close the loop from the post-analytic back to the pre-analytic phase by suggesting the most appropriate examinations for the referring physician’s next patient with a similar problem set. Furthermore, this information can help health care systems identify their most burdensome and needy patients for proactive health care management.
In the United States, the 21st Century Cures Act emphasizes the patient’s role in the post-analytic phase. The program rule on interoperability, information blocking, and Office of National Health Coordinator (ONHC) health IT certification, which implements this act, requires that health care providers give patients access without charge to all the health information in their EHR “without delay.” [27] This legislative directive, predicated on evidence that optimal diagnosis and treatment are enhanced by the convenient availability of patients’ medical records to their physicians, will further drive the aggregation of medical information, regardless of initial source or current repository. This initiative should better enable all physicians involved in a patient’s care to have immediate access to all of that patient’s health care information. Although providing invaluable information to providers, including radiologists and pathologists, the technical and legal demands of this act on provider practices, health systems, and IT vendors will be significant. The requirement that all components of the EHR be promptly provided to the patient, including all laboratory, anatomic pathology, and radiology reports, presents a communication challenge and an opportunity for ID. We will have to modify our reports for patients’ consumption through health care portals, offering the opportunity to integrate and summarize test results for patients and referring physicians.
Notes
This presentation is faithful to the original, with only a few minor changes to presentation, grammar, and punctuation. In some cases important information was missing from the references, and that information was added.