Difference between revisions of "Template:Article of the week"
Shawndouglas (talk | contribs) (Updated article of the week text) |
Shawndouglas (talk | contribs) (Updated article of the week text) |
||
| Line 1: | Line 1: | ||
<div style="float: left; margin: 0.5em 0.9em 0.4em 0em;">[[File: | <div style="float: left; margin: 0.5em 0.9em 0.4em 0em;">[[File:Fig1 Bohn JofLabMed2021 45-6.jpg|240px]]</div> | ||
'''"[[Journal: | '''"[[Journal:Electronic tools in clinical laboratory diagnostics: Key examples, limitations, and value in laboratory medicine|Electronic tools in clinical laboratory diagnostics: Key examples, limitations, and value in laboratory medicine]]"''' | ||
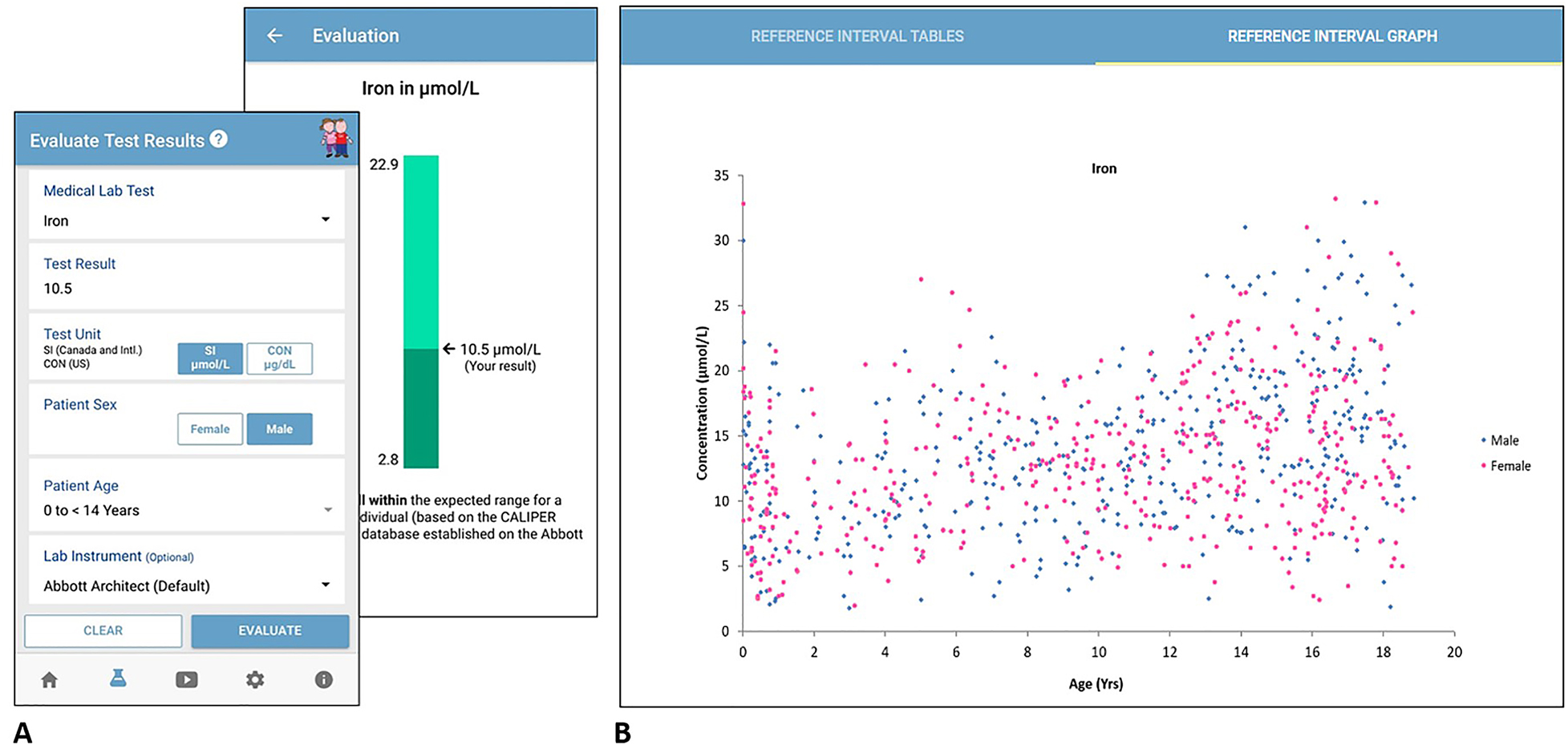
Electronic tools in [[clinical laboratory]] diagnostics can assist [[laboratory]] professionals, clinicians, and patients in medical diagnostic management and laboratory test interpretation. With increasing implementation of [[electronic health record]]s (EHRs) and [[laboratory information system]]s (LIS) worldwide, there is increasing demand for well-designed and evidence-based electronic resources. Both complex data-driven and simple interpretative electronic healthcare tools are currently available to improve the integration of clinical and laboratory [[information]] towards a more patient-centered approach to medicine. Several studies have reported positive clinical impact of electronic healthcare tool implementation in clinical laboratory diagnostics, including in the management of neonatal bilirubinemia, cardiac disease, and nutritional status ... ('''[[Journal:Electronic tools in clinical laboratory diagnostics: Key examples, limitations, and value in laboratory medicine|Full article...]]''')<br /> | |||
<br /> | <br /> | ||
''Recently featured'': | ''Recently featured'': | ||
{{flowlist | | {{flowlist | | ||
* [[Journal:Anatomic pathology quality assurance: Developing an LIS-based tracking and documentation module for intradepartmental consultations|Anatomic pathology quality assurance: Developing an LIS-based tracking and documentation module for intradepartmental consultations]] | |||
* [[Journal:Using knowledge graph structures for semantic interoperability in electronic health records data exchanges|Using knowledge graph structures for semantic interoperability in electronic health records data exchanges]] | * [[Journal:Using knowledge graph structures for semantic interoperability in electronic health records data exchanges|Using knowledge graph structures for semantic interoperability in electronic health records data exchanges]] | ||
* [[Journal:CustodyBlock: A distributed chain of custody evidence framework|CustodyBlock: A distributed chain of custody evidence framework]] | * [[Journal:CustodyBlock: A distributed chain of custody evidence framework|CustodyBlock: A distributed chain of custody evidence framework]] | ||
}} | }} | ||
Revision as of 22:08, 6 February 2023
Electronic tools in clinical laboratory diagnostics can assist laboratory professionals, clinicians, and patients in medical diagnostic management and laboratory test interpretation. With increasing implementation of electronic health records (EHRs) and laboratory information systems (LIS) worldwide, there is increasing demand for well-designed and evidence-based electronic resources. Both complex data-driven and simple interpretative electronic healthcare tools are currently available to improve the integration of clinical and laboratory information towards a more patient-centered approach to medicine. Several studies have reported positive clinical impact of electronic healthcare tool implementation in clinical laboratory diagnostics, including in the management of neonatal bilirubinemia, cardiac disease, and nutritional status ... (Full article...)
Recently featured:
- Anatomic pathology quality assurance: Developing an LIS-based tracking and documentation module for intradepartmental consultations
- Using knowledge graph structures for semantic interoperability in electronic health records data exchanges
- CustodyBlock: A distributed chain of custody evidence framework