Difference between revisions of "Journal:ISO/IEC 17025: History and introduction of concepts"
Shawndouglas (talk | contribs) m (→Notes) |
Shawndouglas (talk | contribs) m (→Notes: Fix) |
||
| (2 intermediate revisions by the same user not shown) | |||
| Line 18: | Line 18: | ||
|website = [https://www.scielo.br/j/qn/a/3gwbfvVs8qydVd5ZstwXCph/ https://www.scielo.br/j/qn/a/3gwbfvVs8qydVd5ZstwXCph/] | |website = [https://www.scielo.br/j/qn/a/3gwbfvVs8qydVd5ZstwXCph/ https://www.scielo.br/j/qn/a/3gwbfvVs8qydVd5ZstwXCph/] | ||
|download = [https://www.scielo.br/j/qn/a/3gwbfvVs8qydVd5ZstwXCph/?format=pdf&lang=en https://www.scielo.br/j/qn/a/3gwbfvVs8qydVd5ZstwXCph/?format=pdf&lang=en] (PDF) | |download = [https://www.scielo.br/j/qn/a/3gwbfvVs8qydVd5ZstwXCph/?format=pdf&lang=en https://www.scielo.br/j/qn/a/3gwbfvVs8qydVd5ZstwXCph/?format=pdf&lang=en] (PDF) | ||
}} | }} | ||
==Abstract== | ==Abstract== | ||
| Line 31: | Line 25: | ||
==Introduction== | ==Introduction== | ||
[[Laboratory|Testing]] and [[Reference laboratory|calibration laboratories]] are organizations which provide measurement results, and important decisions are typically based on these these. Given the great responsibility of these laboratories, being able to assure the [[Quality (business)|quality]] of their service and the reliability of their reported results is important. In order to demonstrate their competence, laboratories throughout the world turn to the [[ISO/IEC 17025]] standard. This document proposes the development and implementation of a [[quality management system]] (QMS) that ensures a high level of [[quality control]] and metrological traceability. Additionally, ISO/IEC 17025 better facilitates access to world markets and provides domestic and international socio-economic benefits.<ref>{{Cite journal |last=Squirrell |first=A. |date=2008-09 |title=Conformity assessment: providing confidence in testing and calibration |url=http://link.springer.com/10.1007/s00769-008-0418-2 |journal=Accreditation and Quality Assurance |language=en |volume=13 |issue=9 |pages=543–546 |doi=10.1007/s00769-008-0418-2 |issn=0949-1775}}</ref> | [[Laboratory|Testing]] and [[Reference laboratory|calibration laboratories]] are organizations which provide measurement results, and important decisions are typically based on these these. Given the great responsibility of these laboratories, being able to assure the [[Quality (business)|quality]] of their service and the reliability of their reported results is important. In order to demonstrate their competence, laboratories throughout the world turn to the [[ISO/IEC 17025]] standard. This document proposes the development and implementation of a [[quality management system]] (QMS) that ensures a high level of [[quality control]] and metrological traceability. Additionally, ISO/IEC 17025 better facilitates access to world markets and provides domestic and international socio-economic benefits.<ref name=":1">{{Cite journal |last=Squirrell |first=A. |date=2008-09 |title=Conformity assessment: providing confidence in testing and calibration |url=http://link.springer.com/10.1007/s00769-008-0418-2 |journal=Accreditation and Quality Assurance |language=en |volume=13 |issue=9 |pages=543–546 |doi=10.1007/s00769-008-0418-2 |issn=0949-1775}}</ref> | ||
For the sake of making knowledge about ISO/IEC 17025 increasingly more accessible, this article presents the concept of quality, how this concept has applied to laboratories over time, the history of ISO/IEC 17025, and details of the QMS proposed by the newest version of this document, published in 2017. | For the sake of making knowledge about ISO/IEC 17025 increasingly more accessible, this article presents the concept of quality, how this concept has applied to laboratories over time, the history of ISO/IEC 17025, and details of the QMS proposed by the newest version of this document, published in 2017. | ||
| Line 40: | Line 34: | ||
Several authors have attempted to define quality. Berhe and Gidey<ref>{{Cite journal |last=Berhe |first=Leakemariam |last2=Gidey |first2=Tesfay |date=2016 |title=Assessing the Awareness and Usage of Quality Control Tools with Emphasis to Statistical Process Control (SPC) in Ethiopian Manufacturing Industries |url=http://www.scirp.org/journal/doi.aspx?DOI=10.4236/iim.2016.86011 |journal=Intelligent Information Management |volume=08 |issue=06 |pages=143–169 |doi=10.4236/iim.2016.86011 |issn=2160-5912}}</ref>, for example, state that the quality of a product is the capacity of such product to meet expectations from both the market and the customer. Quality has also been defined in the perspective of services. Once that service is not a physical matter and, to a certain extent, is intangible, quality has, in this context, two approaches to be assessed: technical quality, which is expressed as the results delivered to the customer, and functional quality, which is expressed as the quality of processes that the customer had to undergo to reach the result.<ref name=":0" /> | Several authors have attempted to define quality. Berhe and Gidey<ref>{{Cite journal |last=Berhe |first=Leakemariam |last2=Gidey |first2=Tesfay |date=2016 |title=Assessing the Awareness and Usage of Quality Control Tools with Emphasis to Statistical Process Control (SPC) in Ethiopian Manufacturing Industries |url=http://www.scirp.org/journal/doi.aspx?DOI=10.4236/iim.2016.86011 |journal=Intelligent Information Management |volume=08 |issue=06 |pages=143–169 |doi=10.4236/iim.2016.86011 |issn=2160-5912}}</ref>, for example, state that the quality of a product is the capacity of such product to meet expectations from both the market and the customer. Quality has also been defined in the perspective of services. Once that service is not a physical matter and, to a certain extent, is intangible, quality has, in this context, two approaches to be assessed: technical quality, which is expressed as the results delivered to the customer, and functional quality, which is expressed as the quality of processes that the customer had to undergo to reach the result.<ref name=":0" /> | ||
Such study about the concepts of quality and their development are useful to define "quality management" more precisely, establishing it as a field of knowledge with its own line of research. Quality management is considered a management system which seeks improvement for products and processes by using the organization’s own knowledge and resources. Its main objectives are satisfying customer requirements and meeting their expectations. | Such study about the concepts of quality and their development are useful to define "quality management" more precisely, establishing it as a field of knowledge with its own line of research. Quality management is considered a management system which seeks improvement for products and processes by using the organization’s own knowledge and resources. Its main objectives are satisfying customer requirements and meeting their expectations.<ref name=":2">{{Cite book |last=Olivares, I.R.B. |year=2009 |title=Gestão de qualidade em laboratórios |publisher=Editora Átomo |isbn=978-85-7670-136-1}}</ref> | ||
===Quality in laboratories=== | ===Quality in laboratories=== | ||
Just like other organizations, a laboratory must be concerned with satisfying customers and their expectations by delivering both reliable results and good customer service. Additionally, it must work to assure the quality of its services. | Just like other organizations, a laboratory must be concerned with satisfying customers and their expectations by delivering both reliable results and good customer service. Additionally, it must work to assure the quality of its services.<ref name=":2" /> | ||
Such concern is shown by Staats | Such concern is shown by Staats<ref>{{Cite journal |last=Staats |first=Gotthard |date=1993 |title=Accreditation in analytical laboratories: a critical assessment of its impact on human beings and techniques |url=http://link.springer.com/10.1007/BF00323001 |journal=Fresenius' Journal of Analytical Chemistry |language=en |volume=345 |issue=12 |pages=739–743 |doi=10.1007/BF00323001 |issn=0937-0633}}</ref>, who declares that, in analytical chemistry, it is not enough just being able to [[Data analysis|analyze information]] in order to be of recognized quality. In this case, [[quality assurance]] is unavoidable in a high-level, complex market. The author also stresses the importance of a quality system which can assure suitable [[information management]], the latter contributing to the reliability and traceability of data. | ||
Christelsohn and Meyer | Christelsohn and Meyer<ref>{{Cite journal |last=Christelsohn, M.; Meyer, J.C. |first= |date=1997-02-28 |title=Discussion Forum |url=http://link.springer.com/10.1007/s007690050103 |journal=Accreditation and Quality Assurance |volume=2 |issue=2 |pages=82–85 |doi=10.1007/s007690050103 |issn=0949-1775}}</ref> state how the laboratory’s concern for its customers is important and stress that QMSs are important tools for achieving such a goal. The researchers analyzed the advantages and disadvantages regarding the prevailing norms and pointed out the need for a robust QMS which places more emphasis on the customer, guided by the [[ISO 9000|ISO 9001]] standard. They also mentioned one of ISO/IEC 17025's predecessor documents, DS/EN 45001, responsible for providing criteria for measuring the technical competence of a laboratory. In such a way, the laboratory’s need to assure the quality of its services to its customers is noticeable, regarding both the reliability of its results and the quality of its customer service, thus making the development and implementation of a QMS very appealing. | ||
According to Olivares | According to Olivares<ref name=":2" />, a QMS is defined as systems put in place seeking to ensure products with the same characteristics and services are delivered in a standardized form, in turn ensuring customers’ interests and meeting their expectations. For laboratories, one of the main sources for QMS development and implementation is the ISO/IEC 17025 standard, which was developed by the [[International Organization for Standardization]] (ISO) and the [[International Electrotechnical Commission]] (IEC). | ||
ISO/IEC 17025, whose history shall be detailed further in this work, has been adopted by many laboratories worldwide. Conclusions have since been drawn after sufficient laboratories fully adopted the QMS proposed by the norm, as seen in the work of Dizadji and Anklam. | ISO/IEC 17025, whose history shall be detailed further in this work, has been adopted by many laboratories worldwide. Conclusions have since been drawn after sufficient laboratories fully adopted the QMS proposed by the norm, as seen in the work of Dizadji and Anklam.<ref name=":3">{{Cite journal |last=Dizadji |first=Ferry |last2=Anklam |first2=Elke |date=2004-06-01 |title=Strategic views of accreditation |url=http://link.springer.com/10.1007/s00769-003-0659-z |journal=Accreditation and Quality Assurance |volume=9 |issue=6 |pages=317–322 |doi=10.1007/s00769-003-0659-z |issn=0949-1775}}</ref> Regarding the adoption of ISO/IEC 17025:1999, Dizadji and Anklam<ref name=":3" /> clearly cited the advantages of doing so, such as increased reliability of the laboratory's work, better efficiency and efficacy, improved market opportunities, and greater competitive advantage in comparison to competitors. The authors also stressed the importance of following the proposed QMS to prove the laboratory capable of delivering its services. They also defined "competence" as the laboratory’s capacity to yield reliable results and to meet customers’ needs. | ||
The concerns with quality assurance and the analytical laboratory's requirement to have quality control in order to yield reliable results are pointed out by Masson. | The concerns with quality assurance and the analytical laboratory's requirement to have quality control in order to yield reliable results are pointed out by Masson.<ref>{{Cite journal |last=Masson |first=Pierre |date=2007-07 |title=Quality control techniques for routine analysis with liquid chromatography in laboratories |url=https://linkinghub.elsevier.com/retrieve/pii/S0021967307004426 |journal=Journal of Chromatography A |language=en |volume=1158 |issue=1-2 |pages=168–173 |doi=10.1016/j.chroma.2007.03.003}}</ref> The author mentions the existence of texts which foresee the construction of quality systems, i.e., ISO/IEC 17025. Nevertheless, the author also clearly states that the laboratory should know its own routine and must adjust to the requirements of such systems, which can be adopted and customized to the laboratory's needs. | ||
Concerns about specific conditions written in ISO/IEC 17025 can be noticed in works related to the quality assurance of results provided by laboratories, such as the discussion about total error and measurement uncertainty by Rozet ''et al.'' | Concerns about specific conditions written in ISO/IEC 17025 can be noticed in works related to the quality assurance of results provided by laboratories, such as the discussion about total error and measurement uncertainty by Rozet ''et al.''<ref>{{Cite journal |last=Rozet |first=E. |last2=Marini |first2=R.D. |last3=Ziemons |first3=E. |last4=Hubert |first4=Ph. |last5=Dewé |first5=W. |last6=Rudaz |first6=S. |last7=Boulanger |first7=B. |date=2011-05 |title=Total error and uncertainty: Friends or foes? |url=https://linkinghub.elsevier.com/retrieve/pii/S0165993611000689 |journal=TrAC Trends in Analytical Chemistry |language=en |volume=30 |issue=5 |pages=797–806 |doi=10.1016/j.trac.2010.12.009}}</ref> Another perspective was introduced by Bodnar ''et al.''<ref>{{Cite journal |last=Bodnar |first=Małgorzata |last2=Namieśnik |first2=Jacek |last3=Konieczka |first3=Piotr |date=2013-11 |title=Validation of a sampling procedure |url=https://linkinghub.elsevier.com/retrieve/pii/S0165993613001714 |journal=TrAC Trends in Analytical Chemistry |language=en |volume=51 |pages=117–126 |doi=10.1016/j.trac.2013.06.011}}</ref>, who point out the necessity for improved focus on sampling recommendations, to avoid errors related to the uncertainty estimation of the aforementioned process. | ||
Lastly, in accordance with the trends of normative texts published by international bodies, Wong | Lastly, in accordance with the trends of normative texts published by international bodies, Wong<ref>{{Cite journal |last=Wong |first=Siu-kay |date=2017-04 |title=Risk-based thinking for chemical testing |url=http://link.springer.com/10.1007/s00769-017-1256-x |journal=Accreditation and Quality Assurance |language=en |volume=22 |issue=2 |pages=103–108 |doi=10.1007/s00769-017-1256-x |issn=0949-1775}}</ref> highlights the union of the laboratory’s risk management to the QMS. It aims to create action plans, which in turn allows labs to better handle risks and opportunities. | ||
==A history of ISO/IEC 17025== | ==A history of ISO/IEC 17025== | ||
As mentioned, the ISO/IEC 17025 standard proposes the enactment of a QMS for laboratories which wish to show their competence, and this standard has been adopted by many laboratories around the world. | As mentioned, the ISO/IEC 17025 standard proposes the enactment of a QMS for laboratories which wish to show their competence, and this standard has been adopted by many laboratories around the world.<ref name=":1" /> Currently, ISO/IEC 17025 is on its third version as an actual standard, with its origin found in various ISO "guides" in the last decades of the previous century. ISO Guide 25: ''Guidelines for assessing the technical competence of testing laboratories'' is considered the first document related to the norm in its current version. This document was issued by the International Laboratory Accreditation Cooperation (ILAC) on October 1, 1978.<ref name=":4">{{Cite journal |last=dos Santos, L.L.; Mainier, F.B. |year=2010 |title=A evolução do sistema de gestão da qualidade em laboratórios de ensaio e calibração e a sua importância para as relações comerciais |url=https://silo.tips/download/a-evoluao-do-sistema-de-gestao-da-qualidade-em-laboratorios-de-ensaio-e-calibraa |journal=VI Congresso Nacional de Excelência em Gestão |pages=1–15 |issn=1984-9354}}</ref> ILAC is an international cooperation, whose members are accreditation bodies for laboratories using the most current standards and guidelines, with representatives in more than 70 countries. This cooperation started in October 1977, seeking to develop international cooperation towards improving the laboratory market by promoting acceptance of accredited test and calibration results.<ref>{{Cite web |date=2021 |title=About ILAC |url=https://ilac.org/about-ilac/ |publisher=International Laboratory Accreditation Cooperation |accessdate=February 2021}}</ref> | ||
ISO Guide 25 did not address calibration laboratories, only testing laboratories. In the document, there were general guidelines towards helping laboratories prove their technical competences. Though initially inadequate, the guide allowed evaluation bodies to ask for other requirements other than the ones already stated in the guide’s text. | ISO Guide 25 did not address calibration laboratories, only testing laboratories. In the document, there were general guidelines towards helping laboratories prove their technical competences. Though initially inadequate, the guide allowed evaluation bodies to ask for other requirements other than the ones already stated in the guide’s text.<ref name=":4" /> The requirements stated in ISO Guide 25 touched upon the organization, its staff, their protection, testing and measuring equipment, calibration, test methods and procedures, the work environment, safety, handling of items to be tested, records management, and test reports.<ref name=":4" /> | ||
ISO Guide 25 was replaced by ISO/IEC Guide 25 ''General requirements for the technical competence of testing laboratories'' on December 12, 1982. For the first time, the document presented itself as both an ISO- and IEC-driven document. | ISO Guide 25 was replaced by ISO/IEC Guide 25 ''General requirements for the technical competence of testing laboratories'' on December 12, 1982. For the first time, the document presented itself as both an ISO- and IEC-driven document.<ref name=":4" /> The ISO is an independent, non-governmental, international organization, made of members from 162 countries. It was created in 1947 to ease international coordination and to unify industrial standardization. Nowadays, it has 784 technical committees and subcommittees responsible for the development of international standards.<ref>{{Cite web |date=2021 |title=About Us |url=https://www.iso.org/about-us.html |publisher=International Organization for Standardization |accessdate=February 2021}}</ref> The IEC, created in 1906, is a worldwide organization that creates and publishes international standards in the areas of electrical, electronic, and related technologies. When plausible, both organizations unite to assure the construction of international standards, which are complementary to each other due to the collaboration between correlated professionals.<ref name=":5">{{Cite web |date=2021 |title=History |url=https://www.iec.ch/history |publisher=International Electrotechnical Commission |accessdate=February 2021}}</ref> | ||
While an improvement, ISO/IEC Guide 25 still only addressed testing laboratories, though mentioning in the “Scope and field of application” section that it could also be used by accreditation and certification bodies, as well as governmental and non-governmental bodies, related to the technical competence of laboratories. | While an improvement, ISO/IEC Guide 25 still only addressed testing laboratories, though mentioning in the “Scope and field of application” section that it could also be used by accreditation and certification bodies, as well as governmental and non-governmental bodies, related to the technical competence of laboratories.<ref name=":5" /> The document addressed the same issues addressed in its predecessor<ref name=":4" />, though it must be noted that, when comparing it with ISO Guide 25, the quality system requirement was added to ISO/IEC Guide 25. | ||
International guides only addressed testing laboratories until, in 1990, ISO/IEC Guide 25: ''General requirements for the competence of calibration and testing laboratories'' was published. | International guides only addressed testing laboratories until, in 1990, ISO/IEC Guide 25: ''General requirements for the competence of calibration and testing laboratories'' was published.<ref name=":4" /> This update highlighted the efforts of ISO/CASCO to publish documents that allow laboratory certification to be made based on internationally established documents. The Council Committee on Conformity Assessment (CASCO) is responsible for the document's issuance, gained by consensus from the Committee itself and supported by the ISO and IEC councils. The purpose of such efforts was to provide support for national systems, thus easing bilateral agreements.<ref name=":4" /> The requirements stated in this updated guide touched upon organization and management, quality systems, audits and critical analyses, staff/personnel, facilities and environment, equipment and reference material, measurement and calibration traceability, calibration and test methods, handling of the calibration and test items, records management, and certificates and reports.<ref name=":4" /> The guide also emphasized that by meeting these criteria, laboratories would meet the criteria of the ISO 9000 standard.<ref name=":4" /> | ||
The 1990 ISO/IEC Guide 25 was the last written version of this document as a guide, even though, according to | The 1990 ISO/IEC Guide 25 was the last written version of this document as a guide, even though, according to van de Leemput<ref name=":6">{{Cite journal |last=van de Leemput |first=Peter J. H. A. M. |date=2000-09-05 |title=ISO/IEC 17025 : 1999 - The new Standard for Laboratories |url=http://link.springer.com/10.1007/s007690000203 |journal=Accreditation and Quality Assurance |volume=5 |issue=9 |pages=394–397 |doi=10.1007/s007690000203 |issn=0949-1775}}</ref>, it had already been written in the format of a full standard by using vocabulary like “shall” and “must” instead of “should” and “may.” This document was replaced in 1999 by the first version of the standard, ISO/IEC 17025: ''General requirements for the competence of testing and calibration laboratories''. Prior to its release, a 1993 ISO revision request was made by the European Technical Committee on Conformity Assessment after the guide’s failure to replace the prevailing European document on technical competence and laboratory accreditation. In 1994, CASCO decided in favor of revising the guide to a full standard after a meeting with stakeholders.<ref name=":6" /> In order to successfully move to a standard, the principle components of the new document had to allow laboratories to display their competence, whether they were interested in accreditation or not. However, despite the push for this allowance, only the revised guide’s requirements were used as criteria for accreditation.<ref name=":6" /> | ||
The revision process took about six years, when drafts were written, discussed, and voted on. During the process, it was decided that if IEC approved the document, its prefix would be ISO/IEC. On November 1999, the document got 95% approval rate, being published on December 15, 1999. | The revision process took about six years, when drafts were written, discussed, and voted on. During the process, it was decided that if IEC approved the document, its prefix would be ISO/IEC. On November 1999, the document got 95% approval rate, being published on December 15, 1999.<ref name=":6" /> Also during the process, it was agreed that the revised document’s relation with ISO 9001 should be clear, with no ambiguity, and its text should cover all aspects of ISO 9001. With this provision, a laboratory which met ISO/IEC 17025 requirements would also meet the requirements of ISO 9001.<ref name=":6" /> | ||
The development group decided that the new document’s requirements would be divided in two categories: management requirements and technical requirements. The overall layout of ISO/IEC 17025:1999 was divided as follows: 1. Objective; 2. Normative References; 3. Terms and definitions; 4. Management requirements; 5. Technical requirements; Annex A; Annex B; References. The requirements of item 4, management requirements, addressed the following topics: organization (4.1); quality system (4.2); document control (4.3); review of requests, tenders and contracts (4.4); subcontracting of tests and calibration (4.5); service and supply purchase (4.6); customers’ service (4.7); complaints (4.8); non-compliant tests and/or calibration work control (4.9); corrective action (4.10); preventive action (4.11); record control (4.12); internal audits (4.13); review by management (4.14). The requirements of item 5, technical requirements, addressed the following topics: general (5.1); staff (5.2); facility and environmental conditions (5.3); test and calibration methods and method validation (5.4); equipment (5.5); metrological traceability (5.6); sampling (5.7); handling of test and calibration items (5.8); quality assurance of tests and calibration results (5.9); reporting of results (5.10). | The development group decided that the new document’s requirements would be divided in two categories: management requirements and technical requirements. The overall layout of ISO/IEC 17025:1999 was divided as follows: 1. Objective; 2. Normative References; 3. Terms and definitions; 4. Management requirements; 5. Technical requirements; Annex A; Annex B; References. The requirements of item 4, management requirements, addressed the following topics: organization (4.1); quality system (4.2); document control (4.3); review of requests, tenders and contracts (4.4); subcontracting of tests and calibration (4.5); service and supply purchase (4.6); customers’ service (4.7); complaints (4.8); non-compliant tests and/or calibration work control (4.9); corrective action (4.10); preventive action (4.11); record control (4.12); internal audits (4.13); review by management (4.14). The requirements of item 5, technical requirements, addressed the following topics: general (5.1); staff (5.2); facility and environmental conditions (5.3); test and calibration methods and method validation (5.4); equipment (5.5); metrological traceability (5.6); sampling (5.7); handling of test and calibration items (5.8); quality assurance of tests and calibration results (5.9); reporting of results (5.10). | ||
According to | According to van de Leemput<ref name=":6" />, the ISO 9001 standard was also under revision at the same time, and the publication of its new version was due in 2000. Nevertheless, the ISO/IEC 17025 was issued in 1999 based on the ISO 9001 norm of 1994. Even though the latter norm would be outdated in a short time, ISO/IEC 17025 was published in 1999 due to the large demand for it and the illogicality of being based on an unfinished, future norm. However, the document was revised again in May 2005 to work in the latest changes to ISO 9001. However, there are no fundamental differences between the 1999 and 2005 version of the norm. Minor difference include highlighting the [[Continual improvement process|continuous improvement]] of the QMS; more emphasis on establishing effective communication with the customer; and use of data to assess the performance of the QMS and to identify improvement opportunities.<ref>{{Cite web |last=United Nations Industrial Development Organization |date=2009 |title=Complying with ISO 17025: A practical guidebook for meeting the requirements of laboratory accreditation schemes based on ISO 17025:2005 or equivalent national standards |url=https://www.unido.org/sites/default/files/2010-08/Complying_with_ISO_17025_A_practical_guidebook_0.pdf |format=PDF |publisher=United Nations Industrial Development Organization}}</ref> The 2005 version also highlights that the management requirements are assigned to the management board instead to the managers. Additionally, due to the new focus on the continuous improvement of the QMS, the topic “Improvement” was added to the management requirements. | ||
The 2005 version had the following division: 1. Objective; 2. Normative references; 3. Terms and definitions; 4. Management board requirements; 5. Technical requirements; Annex A; Annex B; References. The requirements of item 4, management board requirements, addressed the following topics: organization (4.1); management system (4.2); document control (4.3); review of requests, tenders and contracts (4.4); subcontracting of tests and calibrations (4.5); service and supply acquisition (4.6); customer service (4.7); complaints (4.8); non-compliant test and/or calibration work control (4.9); improvement (4.10); corrective action (4.11); preventive action (4.12); record control (4.13); internal audits (4.14); review by management board (4.15). The requirements of item 5, technical requirements, addressed the following topics: general (5.1); personnel (5.2); facility and environmental conditions (5.3); test and calibration methods and method validation (5.4); equipment (5.5); metrological traceability (5.6); sampling (5.7); handling of test and calibration items (5.8); quality assurance of test and calibration results (5.9); reporting of results (5.10). | The 2005 version had the following division: 1. Objective; 2. Normative references; 3. Terms and definitions; 4. Management board requirements; 5. Technical requirements; Annex A; Annex B; References. The requirements of item 4, management board requirements, addressed the following topics: organization (4.1); management system (4.2); document control (4.3); review of requests, tenders and contracts (4.4); subcontracting of tests and calibrations (4.5); service and supply acquisition (4.6); customer service (4.7); complaints (4.8); non-compliant test and/or calibration work control (4.9); improvement (4.10); corrective action (4.11); preventive action (4.12); record control (4.13); internal audits (4.14); review by management board (4.15). The requirements of item 5, technical requirements, addressed the following topics: general (5.1); personnel (5.2); facility and environmental conditions (5.3); test and calibration methods and method validation (5.4); equipment (5.5); metrological traceability (5.6); sampling (5.7); handling of test and calibration items (5.8); quality assurance of test and calibration results (5.9); reporting of results (5.10). | ||
A new version of ISO/IEC 17025 was issued in 2017 in order to update and align it to other current norms, including ISO 9001. To this purpose, the new version included requirements for competency, impartiality, and consistent laboratory operation. | A new version of ISO/IEC 17025 was issued in 2017 in order to update and align it to other current norms, including ISO 9001. To this purpose, the new version included requirements for competency, impartiality, and consistent laboratory operation.<ref name=":7">{{Cite web |last=Rede Metrológica de Minas Gerais |date=2018 |title=Interpretação e Aplicação da Norma ABNT NBR ISO/IEC 17025:2017 |url=https://www.rmmg.com.br/sistema-gest%C3%A3o-norma-17025-2017m |publisher=Rede Metrológica de Minas Gerais}}</ref> ISO/IEC 17025:2017 has a different structure compared to the older version, and it is not divided into management requirements and technical requirements. The 2017 version is divided as follows: 1. Scope; 2. Normative references; 3. Terms and definitions; 4. General requirements; 5. Structural requirements; 6. Resource requirements; 7. Process requirements; 8. Management system requirements; Annex A; Annex B; References/bibliography. The 2017 revision is more process-focused instead of the prior versions' procedural focus, decreasing the number of required policies and procedures. The quality manual is now optional, letting the laboratory decide to establish it or not. The division between technical management and quality management was replaced by a more unified focus on a laboratory's general responsibility management.<ref name=":7" /> Section 4 "General requirements" establishes specific requirements for impartiality and for confidentiality, stressing their importance, in consideration that they are not restricted to the laboratory's policies. This version also emphasizes risk management, pointing out the need for risk identification in many of the norm’s requirements.<ref name=":7" /> | ||
The development of the document in all its version and publications are displayed in Table 1. | The development of the document in all its version and publications are displayed in Table 1. | ||
| Line 131: | Line 125: | ||
As previously mentioned, the new version of ISO/IEC 17025, published in 2017, was required to update and align the document to current versions of other norms, including ISO 9001. As such, it is possible to point out some of the main differences between the previous version (2005) and the updated version (2017). | As previously mentioned, the new version of ISO/IEC 17025, published in 2017, was required to update and align the document to current versions of other norms, including ISO 9001. As such, it is possible to point out some of the main differences between the previous version (2005) and the updated version (2017). | ||
The 2017 version presents a definition for “laboratory,” in which it is defined as a body that performs at least one of the three activities that are presented as “laboratory activity”: testing, calibration, and [[Sample (material)|sampling]], followed by testing or calibration (3.6). It is noticeable that sampling is added as a laboratory activity. | The 2017 version presents a definition for “laboratory,” in which it is defined as a body that performs at least one of the three activities that are presented as “laboratory activity”: testing, calibration, and [[Sample (material)|sampling]], followed by testing or calibration (3.6). It is noticeable that sampling is added as a laboratory activity.<ref name=":8">{{Cite web |last=National Association of Testing Authorities |date=April 2018 |title=General Accreditation Guidance: ISO/IEC 17025:2017 Gap analysis |url=https://nata.com.au/files/2021/05/17025-2017-Gap-analysis.pdf |format=PDF |publisher=National Association of Testing Authorities}}</ref><ref name=":9">{{Cite web |last=United Nations Industrial Development Organization |date=2020 |title=Tested & Accepted: Implementing ISO/IEC 17025:2017 |url=https://hub.unido.org/sites/default/files/publications/Guide%20ISO%2017025-2017.pdf |format=PDF |publisher=United Nations Industrial Development Organization}}</ref> | ||
The concepts of impartiality and independence are differentiated (3.1), and the requirements about impartiality (4.1) and confidentiality (4.2) must be aligned with ISO/CASCO orientation. | The concepts of impartiality and independence are differentiated (3.1), and the requirements about impartiality (4.1) and confidentiality (4.2) must be aligned with ISO/CASCO orientation.<ref name=":8" /><ref name=":9" /> Furthermore, risk-based thinking is implemented, in alignment with ISO 9001:2015. This proposes the monitoring of risks associated with impartiality and the laboratory's activities. ISO/IEC 17025:2017 suggest classifying the appointed risks according to seriousness and tracking them with the intention of maintaining them in a controlled fashion (8.5).<ref name=":8" /><ref name=":9" /> | ||
The term “decision rule” was introduced in 2017. The rule states that the laboratory has to define and apply some criteria in order to decide if the obtained result fulfills the requirements, with the aim of effectively attending to the client’s demands (7.1.3). | The term “decision rule” was introduced in 2017. The rule states that the laboratory has to define and apply some criteria in order to decide if the obtained result fulfills the requirements, with the aim of effectively attending to the client’s demands (7.1.3).<ref name=":8" /><ref name=":9" /> | ||
ISO/IEC 17025:2017's requirements now focus on the outcome, ensuring quality work and validity of result, which provides more flexibility to laboratories. Additionally, the requirements deal with the processes of laboratory activities, looking for a consistent approach on the process and driven, in the document, by the necessity of documentation of the laboratory’s requirements, [[Retention period|retention]] of records, and effective communication with people and organizations affected. | ISO/IEC 17025:2017's requirements now focus on the outcome, ensuring quality work and validity of result, which provides more flexibility to laboratories. Additionally, the requirements deal with the processes of laboratory activities, looking for a consistent approach on the process and driven, in the document, by the necessity of documentation of the laboratory’s requirements, [[Retention period|retention]] of records, and effective communication with people and organizations affected.<ref name=":8" /><ref name=":9" /> | ||
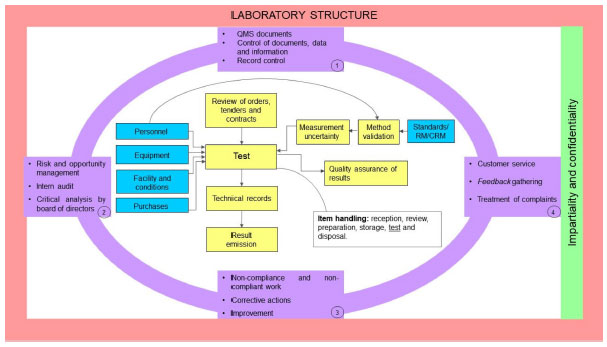
This new version has put attention on technological advances that better enable electronic management of data and information (7.11). | This new version has put attention on technological advances that better enable electronic management of data and information (7.11).<ref name=":9" /> Lastly, the document was restructured, and the requirements are now organized in different sections: 4. General requirements; 5. Structural requirements; 6. Resource requirements; 7. Process requirements, and 8. Management system requirements. The requirements are grouped based on their characteristics. Figure 1 demonstrates how they correlate to each other to create a QMS, with the purpose of meeting all proposed requirements, making the standard’s requirement groups easier to understand. | ||
| Line 159: | Line 153: | ||
Inside the representation of the management processes are the processes directly related to the execution of laboratory activities, which may be rather specific. Figure 1 represents a laboratory whose activities are restricted to tests only, excluding sampling activities. Those processes, represented in blue, correspond to the Section 6 "Resource requirements," and the processes in yellow correspond to Section 7 "Process requirements." These processes are placed in order to clarify how both resource and process requirements correlate to each other. | Inside the representation of the management processes are the processes directly related to the execution of laboratory activities, which may be rather specific. Figure 1 represents a laboratory whose activities are restricted to tests only, excluding sampling activities. Those processes, represented in blue, correspond to the Section 6 "Resource requirements," and the processes in yellow correspond to Section 7 "Process requirements." These processes are placed in order to clarify how both resource and process requirements correlate to each other. | ||
In order to compare the 2017 version with the 2005 version, it can be stated that the management requirements, previously categorized in Section 4, are now | In order to compare the 2017 version with the 2005 version, it can be stated that the management requirements, previously categorized in Section 4, are now reorganized within Sections 4, 5 and 8; and the technical requirements, previously categorized in Section 5, are now reorganized within Sections 6 and 7. | ||
===The value added in ISO/IEC 17025:2017=== | ===The value added in ISO/IEC 17025:2017=== | ||
| Line 178: | Line 172: | ||
==Notes== | ==Notes== | ||
This presentation is faithful to the original, with only a few minor changes to presentation. | This presentation is faithful to the original, with only a few minor changes to presentation. Grammar was tweaked significantly to improve readability. The PMCID, DOI, and article title were also added when they were missing from the original reference. The IEC citation has a broken URL in the original; a suitable substitute for that URL was used for this version. | ||
<!--Place all category tags here--> | <!--Place all category tags here--> | ||
[[Category:LIMSwiki journal articles (added in | [[Category:LIMSwiki journal articles (added in 2023)]] | ||
[[Category:LIMSwiki journal articles (all)]] | [[Category:LIMSwiki journal articles (all)]] | ||
[[Category:LIMSwiki journal articles on quality management]] | [[Category:LIMSwiki journal articles on quality management]] | ||
[[Category:LIMSwiki journal articles on standards]] | [[Category:LIMSwiki journal articles on standards]] | ||
Latest revision as of 18:36, 31 January 2023
| Full article title | ISO/IEC 17025: History and introduction of concepts |
|---|---|
| Journal | Química Nova |
| Author(s) | Miguel, Anna L.R.; Moreiraa, Renata P.L.; de Oliveira, André F. |
| Author affiliation(s) | Universidade Federal de Viçosa |
| Primary contact | Email: annaluisarmiguel at gmail dot com |
| Year published | 2021 |
| Volume and issue | 44(6) |
| Page(s) | 792–6 |
| DOI | 10.21577/0100-4042.20170726 |
| ISSN | 1678-7064 |
| Distribution license | Creative Commons Attribution 4.0 International |
| Website | https://www.scielo.br/j/qn/a/3gwbfvVs8qydVd5ZstwXCph/ |
| Download | https://www.scielo.br/j/qn/a/3gwbfvVs8qydVd5ZstwXCph/?format=pdf&lang=en (PDF) |
Abstract
Quality is an increasingly present concept nowadays, and meeting the needs of customers who buy and use products and hire services becomes essential. For laboratories, the concept is applied not only to the reliability and traceability of the results produced, but it also presents itself in meeting the customer’s needs and providing confidence when signing agreements in the international trade. The concept of quality in a laboratory can be carried out from the development and implementation of a quality management system (QMS). To this end, the normative, internationally accepted document ISO/IEC 17025 aims at instructing the development and implementation of a management system, which ideally proves the technical capacity of testing and calibration laboratories and guides the generation of reliable results. The document is in its third version as a standard, the most current one published in 2017, and it presents requirements to achieve the proposed objective of quality assurance. In the face of the importance of these concepts and the unquestionable need of laboratories to provide reliable and traceable results, this article presents the normative history of ISO/IEC 17025 and its most recent changes. Its intent is to support laboratories whose objective is to implement a QMS according to this normative reference.
Keywords: quality management system, quality assurance, laboratory accreditation, traceability, reliable results
Introduction
Testing and calibration laboratories are organizations which provide measurement results, and important decisions are typically based on these these. Given the great responsibility of these laboratories, being able to assure the quality of their service and the reliability of their reported results is important. In order to demonstrate their competence, laboratories throughout the world turn to the ISO/IEC 17025 standard. This document proposes the development and implementation of a quality management system (QMS) that ensures a high level of quality control and metrological traceability. Additionally, ISO/IEC 17025 better facilitates access to world markets and provides domestic and international socio-economic benefits.[1]
For the sake of making knowledge about ISO/IEC 17025 increasingly more accessible, this article presents the concept of quality, how this concept has applied to laboratories over time, the history of ISO/IEC 17025, and details of the QMS proposed by the newest version of this document, published in 2017.
Definition of quality
In daily life, the search for products and services that fulfil customers’ expectations is always ongoing. As such, the topic of quality is noticeable and any absence of quality is readily perceived. Quality impacts the success of organizations and the lives of every person in a positive manner. Despite being easily identified, perceptions of quality do not rely on a clear definition of what quality is.[2]
Several authors have attempted to define quality. Berhe and Gidey[3], for example, state that the quality of a product is the capacity of such product to meet expectations from both the market and the customer. Quality has also been defined in the perspective of services. Once that service is not a physical matter and, to a certain extent, is intangible, quality has, in this context, two approaches to be assessed: technical quality, which is expressed as the results delivered to the customer, and functional quality, which is expressed as the quality of processes that the customer had to undergo to reach the result.[2]
Such study about the concepts of quality and their development are useful to define "quality management" more precisely, establishing it as a field of knowledge with its own line of research. Quality management is considered a management system which seeks improvement for products and processes by using the organization’s own knowledge and resources. Its main objectives are satisfying customer requirements and meeting their expectations.[4]
Quality in laboratories
Just like other organizations, a laboratory must be concerned with satisfying customers and their expectations by delivering both reliable results and good customer service. Additionally, it must work to assure the quality of its services.[4]
Such concern is shown by Staats[5], who declares that, in analytical chemistry, it is not enough just being able to analyze information in order to be of recognized quality. In this case, quality assurance is unavoidable in a high-level, complex market. The author also stresses the importance of a quality system which can assure suitable information management, the latter contributing to the reliability and traceability of data.
Christelsohn and Meyer[6] state how the laboratory’s concern for its customers is important and stress that QMSs are important tools for achieving such a goal. The researchers analyzed the advantages and disadvantages regarding the prevailing norms and pointed out the need for a robust QMS which places more emphasis on the customer, guided by the ISO 9001 standard. They also mentioned one of ISO/IEC 17025's predecessor documents, DS/EN 45001, responsible for providing criteria for measuring the technical competence of a laboratory. In such a way, the laboratory’s need to assure the quality of its services to its customers is noticeable, regarding both the reliability of its results and the quality of its customer service, thus making the development and implementation of a QMS very appealing.
According to Olivares[4], a QMS is defined as systems put in place seeking to ensure products with the same characteristics and services are delivered in a standardized form, in turn ensuring customers’ interests and meeting their expectations. For laboratories, one of the main sources for QMS development and implementation is the ISO/IEC 17025 standard, which was developed by the International Organization for Standardization (ISO) and the International Electrotechnical Commission (IEC).
ISO/IEC 17025, whose history shall be detailed further in this work, has been adopted by many laboratories worldwide. Conclusions have since been drawn after sufficient laboratories fully adopted the QMS proposed by the norm, as seen in the work of Dizadji and Anklam.[7] Regarding the adoption of ISO/IEC 17025:1999, Dizadji and Anklam[7] clearly cited the advantages of doing so, such as increased reliability of the laboratory's work, better efficiency and efficacy, improved market opportunities, and greater competitive advantage in comparison to competitors. The authors also stressed the importance of following the proposed QMS to prove the laboratory capable of delivering its services. They also defined "competence" as the laboratory’s capacity to yield reliable results and to meet customers’ needs.
The concerns with quality assurance and the analytical laboratory's requirement to have quality control in order to yield reliable results are pointed out by Masson.[8] The author mentions the existence of texts which foresee the construction of quality systems, i.e., ISO/IEC 17025. Nevertheless, the author also clearly states that the laboratory should know its own routine and must adjust to the requirements of such systems, which can be adopted and customized to the laboratory's needs.
Concerns about specific conditions written in ISO/IEC 17025 can be noticed in works related to the quality assurance of results provided by laboratories, such as the discussion about total error and measurement uncertainty by Rozet et al.[9] Another perspective was introduced by Bodnar et al.[10], who point out the necessity for improved focus on sampling recommendations, to avoid errors related to the uncertainty estimation of the aforementioned process.
Lastly, in accordance with the trends of normative texts published by international bodies, Wong[11] highlights the union of the laboratory’s risk management to the QMS. It aims to create action plans, which in turn allows labs to better handle risks and opportunities.
A history of ISO/IEC 17025
As mentioned, the ISO/IEC 17025 standard proposes the enactment of a QMS for laboratories which wish to show their competence, and this standard has been adopted by many laboratories around the world.[1] Currently, ISO/IEC 17025 is on its third version as an actual standard, with its origin found in various ISO "guides" in the last decades of the previous century. ISO Guide 25: Guidelines for assessing the technical competence of testing laboratories is considered the first document related to the norm in its current version. This document was issued by the International Laboratory Accreditation Cooperation (ILAC) on October 1, 1978.[12] ILAC is an international cooperation, whose members are accreditation bodies for laboratories using the most current standards and guidelines, with representatives in more than 70 countries. This cooperation started in October 1977, seeking to develop international cooperation towards improving the laboratory market by promoting acceptance of accredited test and calibration results.[13]
ISO Guide 25 did not address calibration laboratories, only testing laboratories. In the document, there were general guidelines towards helping laboratories prove their technical competences. Though initially inadequate, the guide allowed evaluation bodies to ask for other requirements other than the ones already stated in the guide’s text.[12] The requirements stated in ISO Guide 25 touched upon the organization, its staff, their protection, testing and measuring equipment, calibration, test methods and procedures, the work environment, safety, handling of items to be tested, records management, and test reports.[12]
ISO Guide 25 was replaced by ISO/IEC Guide 25 General requirements for the technical competence of testing laboratories on December 12, 1982. For the first time, the document presented itself as both an ISO- and IEC-driven document.[12] The ISO is an independent, non-governmental, international organization, made of members from 162 countries. It was created in 1947 to ease international coordination and to unify industrial standardization. Nowadays, it has 784 technical committees and subcommittees responsible for the development of international standards.[14] The IEC, created in 1906, is a worldwide organization that creates and publishes international standards in the areas of electrical, electronic, and related technologies. When plausible, both organizations unite to assure the construction of international standards, which are complementary to each other due to the collaboration between correlated professionals.[15]
While an improvement, ISO/IEC Guide 25 still only addressed testing laboratories, though mentioning in the “Scope and field of application” section that it could also be used by accreditation and certification bodies, as well as governmental and non-governmental bodies, related to the technical competence of laboratories.[15] The document addressed the same issues addressed in its predecessor[12], though it must be noted that, when comparing it with ISO Guide 25, the quality system requirement was added to ISO/IEC Guide 25.
International guides only addressed testing laboratories until, in 1990, ISO/IEC Guide 25: General requirements for the competence of calibration and testing laboratories was published.[12] This update highlighted the efforts of ISO/CASCO to publish documents that allow laboratory certification to be made based on internationally established documents. The Council Committee on Conformity Assessment (CASCO) is responsible for the document's issuance, gained by consensus from the Committee itself and supported by the ISO and IEC councils. The purpose of such efforts was to provide support for national systems, thus easing bilateral agreements.[12] The requirements stated in this updated guide touched upon organization and management, quality systems, audits and critical analyses, staff/personnel, facilities and environment, equipment and reference material, measurement and calibration traceability, calibration and test methods, handling of the calibration and test items, records management, and certificates and reports.[12] The guide also emphasized that by meeting these criteria, laboratories would meet the criteria of the ISO 9000 standard.[12]
The 1990 ISO/IEC Guide 25 was the last written version of this document as a guide, even though, according to van de Leemput[16], it had already been written in the format of a full standard by using vocabulary like “shall” and “must” instead of “should” and “may.” This document was replaced in 1999 by the first version of the standard, ISO/IEC 17025: General requirements for the competence of testing and calibration laboratories. Prior to its release, a 1993 ISO revision request was made by the European Technical Committee on Conformity Assessment after the guide’s failure to replace the prevailing European document on technical competence and laboratory accreditation. In 1994, CASCO decided in favor of revising the guide to a full standard after a meeting with stakeholders.[16] In order to successfully move to a standard, the principle components of the new document had to allow laboratories to display their competence, whether they were interested in accreditation or not. However, despite the push for this allowance, only the revised guide’s requirements were used as criteria for accreditation.[16]
The revision process took about six years, when drafts were written, discussed, and voted on. During the process, it was decided that if IEC approved the document, its prefix would be ISO/IEC. On November 1999, the document got 95% approval rate, being published on December 15, 1999.[16] Also during the process, it was agreed that the revised document’s relation with ISO 9001 should be clear, with no ambiguity, and its text should cover all aspects of ISO 9001. With this provision, a laboratory which met ISO/IEC 17025 requirements would also meet the requirements of ISO 9001.[16]
The development group decided that the new document’s requirements would be divided in two categories: management requirements and technical requirements. The overall layout of ISO/IEC 17025:1999 was divided as follows: 1. Objective; 2. Normative References; 3. Terms and definitions; 4. Management requirements; 5. Technical requirements; Annex A; Annex B; References. The requirements of item 4, management requirements, addressed the following topics: organization (4.1); quality system (4.2); document control (4.3); review of requests, tenders and contracts (4.4); subcontracting of tests and calibration (4.5); service and supply purchase (4.6); customers’ service (4.7); complaints (4.8); non-compliant tests and/or calibration work control (4.9); corrective action (4.10); preventive action (4.11); record control (4.12); internal audits (4.13); review by management (4.14). The requirements of item 5, technical requirements, addressed the following topics: general (5.1); staff (5.2); facility and environmental conditions (5.3); test and calibration methods and method validation (5.4); equipment (5.5); metrological traceability (5.6); sampling (5.7); handling of test and calibration items (5.8); quality assurance of tests and calibration results (5.9); reporting of results (5.10).
According to van de Leemput[16], the ISO 9001 standard was also under revision at the same time, and the publication of its new version was due in 2000. Nevertheless, the ISO/IEC 17025 was issued in 1999 based on the ISO 9001 norm of 1994. Even though the latter norm would be outdated in a short time, ISO/IEC 17025 was published in 1999 due to the large demand for it and the illogicality of being based on an unfinished, future norm. However, the document was revised again in May 2005 to work in the latest changes to ISO 9001. However, there are no fundamental differences between the 1999 and 2005 version of the norm. Minor difference include highlighting the continuous improvement of the QMS; more emphasis on establishing effective communication with the customer; and use of data to assess the performance of the QMS and to identify improvement opportunities.[17] The 2005 version also highlights that the management requirements are assigned to the management board instead to the managers. Additionally, due to the new focus on the continuous improvement of the QMS, the topic “Improvement” was added to the management requirements.
The 2005 version had the following division: 1. Objective; 2. Normative references; 3. Terms and definitions; 4. Management board requirements; 5. Technical requirements; Annex A; Annex B; References. The requirements of item 4, management board requirements, addressed the following topics: organization (4.1); management system (4.2); document control (4.3); review of requests, tenders and contracts (4.4); subcontracting of tests and calibrations (4.5); service and supply acquisition (4.6); customer service (4.7); complaints (4.8); non-compliant test and/or calibration work control (4.9); improvement (4.10); corrective action (4.11); preventive action (4.12); record control (4.13); internal audits (4.14); review by management board (4.15). The requirements of item 5, technical requirements, addressed the following topics: general (5.1); personnel (5.2); facility and environmental conditions (5.3); test and calibration methods and method validation (5.4); equipment (5.5); metrological traceability (5.6); sampling (5.7); handling of test and calibration items (5.8); quality assurance of test and calibration results (5.9); reporting of results (5.10).
A new version of ISO/IEC 17025 was issued in 2017 in order to update and align it to other current norms, including ISO 9001. To this purpose, the new version included requirements for competency, impartiality, and consistent laboratory operation.[18] ISO/IEC 17025:2017 has a different structure compared to the older version, and it is not divided into management requirements and technical requirements. The 2017 version is divided as follows: 1. Scope; 2. Normative references; 3. Terms and definitions; 4. General requirements; 5. Structural requirements; 6. Resource requirements; 7. Process requirements; 8. Management system requirements; Annex A; Annex B; References/bibliography. The 2017 revision is more process-focused instead of the prior versions' procedural focus, decreasing the number of required policies and procedures. The quality manual is now optional, letting the laboratory decide to establish it or not. The division between technical management and quality management was replaced by a more unified focus on a laboratory's general responsibility management.[18] Section 4 "General requirements" establishes specific requirements for impartiality and for confidentiality, stressing their importance, in consideration that they are not restricted to the laboratory's policies. This version also emphasizes risk management, pointing out the need for risk identification in many of the norm’s requirements.[18]
The development of the document in all its version and publications are displayed in Table 1.
| ||||||||||||||||||||||||||||||||
ISO/IEC 17025:2017
As previously mentioned, the new version of ISO/IEC 17025, published in 2017, was required to update and align the document to current versions of other norms, including ISO 9001. As such, it is possible to point out some of the main differences between the previous version (2005) and the updated version (2017).
The 2017 version presents a definition for “laboratory,” in which it is defined as a body that performs at least one of the three activities that are presented as “laboratory activity”: testing, calibration, and sampling, followed by testing or calibration (3.6). It is noticeable that sampling is added as a laboratory activity.[19][20]
The concepts of impartiality and independence are differentiated (3.1), and the requirements about impartiality (4.1) and confidentiality (4.2) must be aligned with ISO/CASCO orientation.[19][20] Furthermore, risk-based thinking is implemented, in alignment with ISO 9001:2015. This proposes the monitoring of risks associated with impartiality and the laboratory's activities. ISO/IEC 17025:2017 suggest classifying the appointed risks according to seriousness and tracking them with the intention of maintaining them in a controlled fashion (8.5).[19][20]
The term “decision rule” was introduced in 2017. The rule states that the laboratory has to define and apply some criteria in order to decide if the obtained result fulfills the requirements, with the aim of effectively attending to the client’s demands (7.1.3).[19][20]
ISO/IEC 17025:2017's requirements now focus on the outcome, ensuring quality work and validity of result, which provides more flexibility to laboratories. Additionally, the requirements deal with the processes of laboratory activities, looking for a consistent approach on the process and driven, in the document, by the necessity of documentation of the laboratory’s requirements, retention of records, and effective communication with people and organizations affected.[19][20]
This new version has put attention on technological advances that better enable electronic management of data and information (7.11).[20] Lastly, the document was restructured, and the requirements are now organized in different sections: 4. General requirements; 5. Structural requirements; 6. Resource requirements; 7. Process requirements, and 8. Management system requirements. The requirements are grouped based on their characteristics. Figure 1 demonstrates how they correlate to each other to create a QMS, with the purpose of meeting all proposed requirements, making the standard’s requirement groups easier to understand.
|
The requirements which belong to Section 5 "Structural requirements" address the aspects that make the laboratory capable of doing its activities, the latter being considered the base of the QMS. In Figure 1, the laboratory structure is represented in a way that it encompasses all requirements left. The next group to be represented is Section 4 "General requirements" which addresses impartiality and confidentiality in the development of the laboratory's activities, setting up the risk to impartiality management in a continuous manner. In Figure 1, this section is located with the structural requirements group.
After the definition of the QMS' basic parts, the management processes are represented. They guide and encompass all laboratory activities developed by the laboratory. In Figure 1, these processes are represented in lilac, are separated in four blocks, and encircle the remaining requirements. These processes must be executed in the same manner for all tests addressed by the QMS, while other requirements might be specific depending on the laboratory activity. Most of these processes are requirements of Section 8 "Management requirements" and include some requirements of Section 7 "Process requirements."
Inside the representation of the management processes are the processes directly related to the execution of laboratory activities, which may be rather specific. Figure 1 represents a laboratory whose activities are restricted to tests only, excluding sampling activities. Those processes, represented in blue, correspond to the Section 6 "Resource requirements," and the processes in yellow correspond to Section 7 "Process requirements." These processes are placed in order to clarify how both resource and process requirements correlate to each other.
In order to compare the 2017 version with the 2005 version, it can be stated that the management requirements, previously categorized in Section 4, are now reorganized within Sections 4, 5 and 8; and the technical requirements, previously categorized in Section 5, are now reorganized within Sections 6 and 7.
The value added in ISO/IEC 17025:2017
Given all the main changes between the 2005 and 2017 versions, it is concluded that the new version of the standard proposes a more efficient management system, reducing the number of mandatory procedures and not requiring a quality manual. It instead focuses on consistent processes, with personnel able to perform them and maintain objective evidence of activities duly recorded. This new proposition may help laboratories to create and implement a QMS consistent with its own reality, according to the size of the staff of each laboratory and the activities it pursues (i.e., testing or calibration, in multiple areas such as environment, forensics, food, etc.). Also, the concept of risk management is proposed to help laboratories assure the quality of their activities once the risk to laboratory activities and impartiality are duly monitored and treated.
In addition, this new version highlights the importance of meeting the demands of customers through the adoption of a decision rule to report a final result. Focusing on the importance of the measurement provided by the laboratory to the customer's interests, this position reinforces the importance of laboratories in providing reliable and traceable results to support decision making in different situations.
In this way, ISO/IEC 17025:2017 proposes the development and implementation of a QMS through the requirements established and mentioned above to prove the technical competence and ensure the quality of the results produced by the laboratory. The use of this standard can be justified as a tool to assist the implementation of quality in testing and calibration laboratories.
Conclusions
Since the primary service provided by testing and calibration laboratories, providing analytical results, is largely used to support decision-making, with its own social impact, these laboratories must be aware of and put to use tools to ensure reliable, quality analytical results are generated. As shown, the QMS proposed by ISO/IEC 17025 contributes to the establishment of a set of laboratory practices based on quality assurance. By enacting ISO/IEC 17025, testing and calibration laboratories demonstrate they are responsible with their activities and their impacts, and put quality management and metrological traceability at the forefront of their operations.
Acknowledgements
The authors are grateful to the Brazilian Agency for financial support: Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG).
References
- ↑ 1.0 1.1 Squirrell, A. (1 September 2008). "Conformity assessment: providing confidence in testing and calibration" (in en). Accreditation and Quality Assurance 13 (9): 543–546. doi:10.1007/s00769-008-0418-2. ISSN 0949-1775. http://link.springer.com/10.1007/s00769-008-0418-2.
- ↑ 2.0 2.1 Gomes, P.J.P (2004). "A evolução do conceito de qualidade: dos bens manufacturados aos serviços de informação". Cadernos BAD 2 (6): 6–18. http://eprints.rclis.org/10401/1/GomesBAD204.pdf?&hl=pt-BR&sa=X&scisig=AAGBfm0o4Qp_QZUtLm-GkpB6D0CsMpReFQ&nossl=1&oi=scholarr&ei=pZoRVZGALrCBsQT_3ICIAg&ved=0CBsQgAMoADAA.
- ↑ Berhe, Leakemariam; Gidey, Tesfay (2016). "Assessing the Awareness and Usage of Quality Control Tools with Emphasis to Statistical Process Control (SPC) in Ethiopian Manufacturing Industries". Intelligent Information Management 08 (06): 143–169. doi:10.4236/iim.2016.86011. ISSN 2160-5912. http://www.scirp.org/journal/doi.aspx?DOI=10.4236/iim.2016.86011.
- ↑ 4.0 4.1 4.2 Olivares, I.R.B. (2009). Gestão de qualidade em laboratórios. Editora Átomo. ISBN 978-85-7670-136-1.
- ↑ Staats, Gotthard (1993). "Accreditation in analytical laboratories: a critical assessment of its impact on human beings and techniques" (in en). Fresenius' Journal of Analytical Chemistry 345 (12): 739–743. doi:10.1007/BF00323001. ISSN 0937-0633. http://link.springer.com/10.1007/BF00323001.
- ↑ Christelsohn, M.; Meyer, J.C. (28 February 1997). "Discussion Forum". Accreditation and Quality Assurance 2 (2): 82–85. doi:10.1007/s007690050103. ISSN 0949-1775. http://link.springer.com/10.1007/s007690050103.
- ↑ 7.0 7.1 Dizadji, Ferry; Anklam, Elke (1 June 2004). "Strategic views of accreditation". Accreditation and Quality Assurance 9 (6): 317–322. doi:10.1007/s00769-003-0659-z. ISSN 0949-1775. http://link.springer.com/10.1007/s00769-003-0659-z.
- ↑ Masson, Pierre (1 July 2007). "Quality control techniques for routine analysis with liquid chromatography in laboratories" (in en). Journal of Chromatography A 1158 (1-2): 168–173. doi:10.1016/j.chroma.2007.03.003. https://linkinghub.elsevier.com/retrieve/pii/S0021967307004426.
- ↑ Rozet, E.; Marini, R.D.; Ziemons, E.; Hubert, Ph.; Dewé, W.; Rudaz, S.; Boulanger, B. (1 May 2011). "Total error and uncertainty: Friends or foes?" (in en). TrAC Trends in Analytical Chemistry 30 (5): 797–806. doi:10.1016/j.trac.2010.12.009. https://linkinghub.elsevier.com/retrieve/pii/S0165993611000689.
- ↑ Bodnar, Małgorzata; Namieśnik, Jacek; Konieczka, Piotr (1 November 2013). "Validation of a sampling procedure" (in en). TrAC Trends in Analytical Chemistry 51: 117–126. doi:10.1016/j.trac.2013.06.011. https://linkinghub.elsevier.com/retrieve/pii/S0165993613001714.
- ↑ Wong, Siu-kay (1 April 2017). "Risk-based thinking for chemical testing" (in en). Accreditation and Quality Assurance 22 (2): 103–108. doi:10.1007/s00769-017-1256-x. ISSN 0949-1775. http://link.springer.com/10.1007/s00769-017-1256-x.
- ↑ 12.0 12.1 12.2 12.3 12.4 12.5 12.6 12.7 12.8 dos Santos, L.L.; Mainier, F.B. (2010). "A evolução do sistema de gestão da qualidade em laboratórios de ensaio e calibração e a sua importância para as relações comerciais". VI Congresso Nacional de Excelência em Gestão: 1–15. ISSN 1984-9354. https://silo.tips/download/a-evoluao-do-sistema-de-gestao-da-qualidade-em-laboratorios-de-ensaio-e-calibraa.
- ↑ "About ILAC". International Laboratory Accreditation Cooperation. 2021. https://ilac.org/about-ilac/. Retrieved February 2021.
- ↑ "About Us". International Organization for Standardization. 2021. https://www.iso.org/about-us.html. Retrieved February 2021.
- ↑ 15.0 15.1 "History". International Electrotechnical Commission. 2021. https://www.iec.ch/history. Retrieved February 2021.
- ↑ 16.0 16.1 16.2 16.3 16.4 16.5 van de Leemput, Peter J. H. A. M. (5 September 2000). "ISO/IEC 17025 : 1999 - The new Standard for Laboratories". Accreditation and Quality Assurance 5 (9): 394–397. doi:10.1007/s007690000203. ISSN 0949-1775. http://link.springer.com/10.1007/s007690000203.
- ↑ United Nations Industrial Development Organization (2009). "Complying with ISO 17025: A practical guidebook for meeting the requirements of laboratory accreditation schemes based on ISO 17025:2005 or equivalent national standards" (PDF). United Nations Industrial Development Organization. https://www.unido.org/sites/default/files/2010-08/Complying_with_ISO_17025_A_practical_guidebook_0.pdf.
- ↑ 18.0 18.1 18.2 Rede Metrológica de Minas Gerais (2018). "Interpretação e Aplicação da Norma ABNT NBR ISO/IEC 17025:2017". Rede Metrológica de Minas Gerais. https://www.rmmg.com.br/sistema-gest%C3%A3o-norma-17025-2017m.
- ↑ 19.0 19.1 19.2 19.3 19.4 National Association of Testing Authorities (April 2018). "General Accreditation Guidance: ISO/IEC 17025:2017 Gap analysis" (PDF). National Association of Testing Authorities. https://nata.com.au/files/2021/05/17025-2017-Gap-analysis.pdf.
- ↑ 20.0 20.1 20.2 20.3 20.4 20.5 United Nations Industrial Development Organization (2020). "Tested & Accepted: Implementing ISO/IEC 17025:2017" (PDF). United Nations Industrial Development Organization. https://hub.unido.org/sites/default/files/publications/Guide%20ISO%2017025-2017.pdf.
Notes
This presentation is faithful to the original, with only a few minor changes to presentation. Grammar was tweaked significantly to improve readability. The PMCID, DOI, and article title were also added when they were missing from the original reference. The IEC citation has a broken URL in the original; a suitable substitute for that URL was used for this version.