Difference between revisions of "Journal:Strategies for laboratory professionals to drive laboratory stewardship"
Shawndouglas (talk | contribs) (Saving and adding more.) |
Shawndouglas (talk | contribs) (Saving and adding more.) |
||
| Line 60: | Line 60: | ||
National medical societies such as the ASCP that joined the Choosing Wisely campaign initially identified five tests or procedures often used in their specialty whose use, at least in some situations, should be reconsidered. Numerous Choosing Wisely guidelines that focus on laboratory medicine topics now exist, being submitted by laboratory societies (e.g., ASCP, the American Association of Blood Banks, the American Society for Clinical Laboratory Science, the American Society for Microbiology) and by specialty societies that use laboratory tests (e.g., American College of Obstetricians and Gynecologists, American Academy of Family Physicians).<ref name="ABIMClinList">{{Cite web |last=ABIM Foundation |title=Clinician Lists |work=Choosing Wisely |url=https://www.choosingwisely.org/clinician-lists/ |publisher=ABIM Foundation |accessdate=30 March 2021}}</ref><ref name="WaibelSys18">{{Cite journal |last=Waibel |first=Elizabeth |last2=Garcia |first2=Edna |last3=Kelly |first3=Melissa |last4=Soles |first4=Ryan |last5=Hilborne |first5=Lee |date=2018-02-17 |title=Systematic Review of Non-ASCP Choosing Wisely Recommendations Relevant to Pathology and Laboratory Medicine |url=https://academic.oup.com/ajcp/article/149/3/267/4841631 |journal=American Journal of Clinical Pathology |language=en |volume=149 |issue=3 |pages=267–274 |doi=10.1093/ajcp/aqx159 |issn=0002-9173}}</ref> For example, in August 2019 the American Academy of Pediatrics recommended not testing for Lyme disease as a cause of musculoskeletal symptoms without an exposure history or appropriate exam findings. They noted: “The musculoskeletal manifestations of Lyme disease include brief attacks of arthralgia with early disseminated Lyme and/or intermittent or persistent episodes of arthritis in one or a few large joints, with predilection for the knee, in late disease. Lyme testing in the absence of these features and without appropriate exposure from living in or traveling to a Lyme endemic area increases the likelihood of false positive results and may lead to unnecessary follow-up and therapy. Diffuse arthralgias, myalgias, or fibromyalgia alone are not criteria for musculoskeletal Lyme disease.”<ref name="AAPDoNot19">{{Cite web |last=American Academy of Pediatrics |date=06 August 2019 |title=Do not test for Lyme disease as a cause of musculoskeletal symptoms without an exposure history or appropriate exam findings. |work=Choosing Wisely |url=https://www.choosingwisely.org/clinician-lists/aap-sorh-testing-for-lyme-disease-with-musculoskeletal-symptoms/ |publisher=ABIM Foundation |accessdate=30 March 2021}}</ref> | National medical societies such as the ASCP that joined the Choosing Wisely campaign initially identified five tests or procedures often used in their specialty whose use, at least in some situations, should be reconsidered. Numerous Choosing Wisely guidelines that focus on laboratory medicine topics now exist, being submitted by laboratory societies (e.g., ASCP, the American Association of Blood Banks, the American Society for Clinical Laboratory Science, the American Society for Microbiology) and by specialty societies that use laboratory tests (e.g., American College of Obstetricians and Gynecologists, American Academy of Family Physicians).<ref name="ABIMClinList">{{Cite web |last=ABIM Foundation |title=Clinician Lists |work=Choosing Wisely |url=https://www.choosingwisely.org/clinician-lists/ |publisher=ABIM Foundation |accessdate=30 March 2021}}</ref><ref name="WaibelSys18">{{Cite journal |last=Waibel |first=Elizabeth |last2=Garcia |first2=Edna |last3=Kelly |first3=Melissa |last4=Soles |first4=Ryan |last5=Hilborne |first5=Lee |date=2018-02-17 |title=Systematic Review of Non-ASCP Choosing Wisely Recommendations Relevant to Pathology and Laboratory Medicine |url=https://academic.oup.com/ajcp/article/149/3/267/4841631 |journal=American Journal of Clinical Pathology |language=en |volume=149 |issue=3 |pages=267–274 |doi=10.1093/ajcp/aqx159 |issn=0002-9173}}</ref> For example, in August 2019 the American Academy of Pediatrics recommended not testing for Lyme disease as a cause of musculoskeletal symptoms without an exposure history or appropriate exam findings. They noted: “The musculoskeletal manifestations of Lyme disease include brief attacks of arthralgia with early disseminated Lyme and/or intermittent or persistent episodes of arthritis in one or a few large joints, with predilection for the knee, in late disease. Lyme testing in the absence of these features and without appropriate exposure from living in or traveling to a Lyme endemic area increases the likelihood of false positive results and may lead to unnecessary follow-up and therapy. Diffuse arthralgias, myalgias, or fibromyalgia alone are not criteria for musculoskeletal Lyme disease.”<ref name="AAPDoNot19">{{Cite web |last=American Academy of Pediatrics |date=06 August 2019 |title=Do not test for Lyme disease as a cause of musculoskeletal symptoms without an exposure history or appropriate exam findings. |work=Choosing Wisely |url=https://www.choosingwisely.org/clinician-lists/aap-sorh-testing-for-lyme-disease-with-musculoskeletal-symptoms/ |publisher=ABIM Foundation |accessdate=30 March 2021}}</ref> | ||
==Implementation of a laboratory stewardship program== | |||
Successfully implementing a laboratory stewardship program requires five key elements: a clear vision and organizational alignment, skills, resources, incentives, and a plan of action. Each element, with examples of implementation, is described in detail below. | |||
===A clear vision and organizational alignment=== | |||
Implementing a laboratory stewardship program is an opportunity for laboratory professionals to drive the change they want to see. It is important, however, that the laboratory's vision (i.e., aspirations) is aligned with that of the organization. Absent a clear vision and organizational support, there will be confusion with little chance for meaningful success. Successful program implementation may require a change or alignment of culture (i.e., the values, beliefs, and norms influencing how people behave as members of an organization) and a shared understanding of why change is needed.<ref name="RavasiResp06">{{Cite journal |last=Ravasi |first=Davide |last2=Schultz |first2=Majken |date=2006-06 |title=Responding to Organizational Identity Threats: Exploring the Role of Organizational Culture |url=http://journals.aom.org/doi/10.5465/amj.2006.21794663 |journal=Academy of Management Journal |language=en |volume=49 |issue=3 |pages=433–458 |doi=10.5465/amj.2006.21794663 |issn=0001-4273}}</ref> Critical to this change management is a solid laboratory professional–clinician partnership. | |||
====Understand the health system organization and establish key partnerships with stakeholders==== | |||
When initiating a laboratory stewardship program, one must first understand the health system's organization and culture. Laboratory stewardship is by nature interdisciplinary, and understanding the health system enables identification of key stakeholders across the organization with whom laboratory professionals will need to partner to achieve desired clinical outcomes. Key stakeholders often include health system medical (e.g., physicians, nurses, physician assistants, pharmacists) and administrative (e.g., safety managers, information technology analysts, quality improvement specialists, members of the “C-suite”) leadership in addition to laboratory medical and administrative leadership. | |||
Traditional organizational structures are changing in response to pressures throughout healthcare; now, organizational structures vary such that an understanding of one does not likely extend to an understanding of others. Traditional departmental medical staff structures still maintain important roles, but alternative structures such as service or product lines have developed to provide an interdisciplinary, integrated care approach when managing specific patient populations (e.g., cardiology, oncology). Therefore, understand how physicians, other healthcare providers, and administrators interact to achieve desired system outcomes so that the stewardship initiative can be integrated smoothly. For example, leaders and departments tasked with improving budget and financial scorecards may have specific targets; laboratory initiatives can help them reach their targets. Health systems usually have working committees focused on quality, infection control, medication management, and medical staff privileging. An existing committee may already be tasked with elements of laboratory stewardship, in which case the stewardship initiative should be integrated carefully and with buy-in from these stakeholders. | |||
Given the importance of physician leadership to every laboratory stewardship program, time is needed to understand the medical staff who use laboratory services. Some physicians may be employed; others might be independent or contracted. These relationships may become relevant when selecting program participants. Alignment may be more difficult when physicians are independent because if their income is generated solely through direct patient care, investing many hours on an “extracurricular” endeavor results in lost income, unless the system compensates individuals for time lost from direct patient care. | |||
====Establish leadership and governance for the laboratory stewardship program==== | |||
A laboratory stewardship program requires dedicated oversight to focus on identifying and implementing needed changes. Before establishing the program's oversight structure, however, recognize how the key administrative (e.g., CEO/COO for the system, or the chief technologist in the laboratory) and medical (e.g., CMO/director of medical affairs for the system, or laboratory director/[[Clinical Laboratory Improvement Amendments|CLIA]] certificate holder in the laboratory) leaders intersect in terms of their roles and responsibilities. This is important both to make sure the right people are engaged as initiatives are undertaken and to assure that organizational structures and lines of authority are respected. | |||
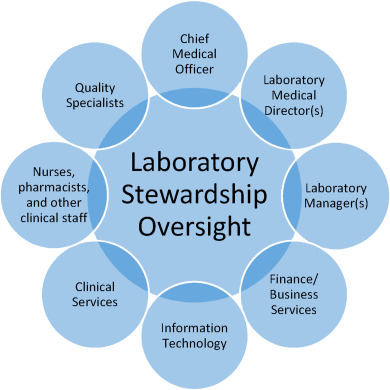
The oversight structure may be a specific committee, or a subcommittee or task force, of an existing health system committee (Fig. 1). Participants in stewardship oversight should include stakeholders from the laboratory, inpatient and ambulatory providers, and administrative leaders.<ref name="DickersonTrans17" /> Laboratory participants may include pathologists, operational directors, and medical laboratory scientists. Clinical participants may include primary care and specialist clinicians and other staff (e.g., nurse practitioners, nurses, and physician assistants). Health system administrators (e.g., finance, information technology, risk management and process improvement) are important to help integrate and align the program with the broader organization. Additional individuals may participate based on their subject matter expertise related to specific program initiatives. | |||
[[File:Fig1 White PractLabMed2021 26.jpg|390px]] | |||
{{clear}} | |||
{| | |||
| STYLE="vertical-align:top;"| | |||
{| border="0" cellpadding="5" cellspacing="0" width="390px" | |||
|- | |||
| style="background-color:white; padding-left:10px; padding-right:10px;"| <blockquote>'''Figure 1.''' Possible structure for a laboratory stewardship oversight committee. Regardless of the oversight structure established in a particular health system, participants in laboratory stewardship oversight should include stakeholders from the laboratory, clinic, and health system administration.</blockquote> | |||
|- | |||
|} | |||
|} | |||
Successful programs have a specific point of accountability (e.g., a designated leader or two co-leaders). There must also be a knowledgeable staff person (e.g., a program manager) specifically assigned to the laboratory stewardship initiative. The staff person must keep the program on track by aggregating data, coordinating analyst and other team member activities, scheduling meetings, engaging subject matter experts, and ensuring the leader or co-leaders are always apprised of activities. | |||
====Communicate a compelling case for change==== | |||
Stakeholders must be convinced of the need for a laboratory stewardship program. Laboratory-based stewardship champions must create enthusiasm to secure non-laboratory stakeholder buy-in to create the true interdisciplinary team. Once non-laboratory stakeholders support the program, they can also become stewardship champions who help others recognize the value of laboratory services and secure additional buy-in for the program. The interdisciplinary approach helps assure initial efforts are integrated, make operational sense, and progress toward achieving desired clinical outcomes. | |||
Laboratory professionals can actively cultivate other stakeholders to become stewardship champions. Laboratory medical and technologist members who serve on organizational committees may already have established relationships and credibility with patient-facing physicians and clinical staff. Look to existing successful laboratory-hospital partnerships such as effective blood utilization and antimicrobial stewardship initiatives. Laboratory stewardship can thus be framed as an extension of existing partnerships. | |||
Compelling case discussions should include [[Data visualization|presentation of data]] (e.g., declining performance on HEDIS indicators) to establish the importance of selected initiatives and provide the basis for change, followed by discussions on desired outcomes and how to achieve them. Peer-to-peer conversations, rather than lectures that come across as patronizing, can effectively educate stakeholders and promote alignment. When aligning on desired outcomes, the focus should be on achieving beneficial patient outcomes and not simply achieving appropriate laboratory utilization (e.g., correct test ordering patterns). This framing is more inclusive, should align with the organization's strategic plan, and highlights a shared value of laboratorians and clinicians. | |||
Discussions allow stakeholders to share diverse perspectives. Ongoing engagement, especially across departments and disciplines, leads to identification of challenges and opportunities that may not otherwise be recognized. Through collaborative discussion, stewardship champions and key stakeholders can begin developing initial project proposals. Initial projects should each have a reasonable scope so that early program initiatives have the best chance to succeed and prove value. Early successes promote further change and should be widely shared across the organization to build program support. The team can then move on to bigger challenges, including looking at changing deeply ingrained practice challenges. | |||
===Skills=== | |||
A laboratory stewardship program requires analytic, subject matter, presentation, project management, interpersonal, and leadership skills. Rarely all the needed skills to carry out a successful laboratory stewardship program will be embodied in one person. That is precisely why an effective team brings multiple colleagues to the table, each with unique skills and abilities, to define, evaluate, and address problems. | |||
====Analytic skills==== | |||
Revision as of 18:33, 5 August 2021
| Full article title | Strategies for laboratory professionals to drive laboratory stewardship |
|---|---|
| Journal | Practical Laboratory Medicine |
| Author(s) | White, Terra, E.; Wong, Wesley B.; Janowiak, Diane; Hilborne, Lee H. |
| Author affiliation(s) | Quest Diagnostics, hc1, University of California - Los Angeles |
| Primary contact | terra dot e dot white at questdiagnostics dot com |
| Year published | 2021 |
| Volume and issue | 26 |
| Article # | e00249 |
| DOI | 10.1016/j.plabm.2021.e00249 |
| ISSN | 2352-5517 |
| Distribution license | Creative Commons Attribution 4.0 International |
| Website | https://www.sciencedirect.com/science/article/pii/S2352551721000494 |
| Download | https://www.sciencedirect.com/science/article/pii/S2352551721000494/pdfft (PDF) |
|
|
This article should be considered a work in progress and incomplete. Consider this article incomplete until this notice is removed. |
Abstract
Appropriate laboratory testing is critical in today's healthcare environment that aims to improve patient care while reducing cost. In recent years, laboratory stewardship has emerged as a strategy for assuring quality in laboratory medicine with the goal of providing the right test for the right patient at the right time. Implementing a laboratory stewardship program now presents a valuable opportunity for laboratory professionals to exercise leadership within health systems and to drive change toward realizing aims in healthcare. The proposed framework for program implementation includes five key elements: 1) a clear vision and organizational alignment; 2) appropriate skills for program execution and management; 3) resources to support the program; 4) incentives to motivate participation; and, 5) a plan of action that articulates program objectives and metrics. This framework builds upon principles of change management, with emphasis on engagement with clinical and administrative stakeholders and the use of clinical data as the basis for change. These strategies enable laboratory professionals to cultivate organizational support for improving laboratory use and take a leading role in providing high-quality patient care.
Keywords: clinical laboratory, laboratory stewardship, quality of health care, change management, patient care, leadership
Introduction
Establishing the correct diagnosis is fundamental to the appropriate treatment of patients, making laboratory testing and other diagnostic services (e.g., radiology) critical to the overall practice of medicine. Some suggest that up to 70% of medical decisions are based, in part, on laboratory findings.[1] While the specific percentage may be debatable, there is no question that laboratory diagnostics are central for medical practice today. The coronavirus disease 2019 (COVID-19) pandemic highlighted the critical role that the nation's laboratories and laboratory professionals play in assuring quality healthcare, well beyond just the pandemic response. Healthcare leaders repeatedly acknowledged the importance of laboratory testing for COVID-19 patient management to, for example, assess symptomatic patients, identify emerging variants, and perform effective contact tracing.[2] Laboratory medicine is also central to the worlds' public health response to COVID-19.[3]
Laboratory testing is typically one of the highest volume medical activities in a health system.[4] Despite their major role in driving clinical decision-making, laboratory diagnostics are highly variable and filled with opportunities to increase appropriate testing while reducing waste. Annual cost estimates in the U.S. for low-value screening, testing, or procedures range from $17.2 billion to $27.9 billion.[5] Excessive diagnostic testing can also increase patient risk while not improving diagnostic certainty.[6] Achieving the aims of higher quality and lower cost depends on the laboratory community taking a central role in improving effective test utilization.
Over the last century, pathologists and laboratory professionals honed their craft while simultaneously expanding tools available to screen, diagnose, treat, and manage patients. The laboratorian historically focused quality efforts on a test's analytic quality. While analytic quality is necessary for appropriate care, it is not sufficient; effective test utilization requires clear medical justification to support the need for a particular test with an understanding of how the results will contribute to guiding patient management. This discussion focuses on the evolution of effective test utilization and implementation of laboratory stewardship as a laboratory leadership strategy to assure effective test use.
Evolution of quality in laboratory medicine
The pursuit of laboratory medicine quality improvement will shortly celebrate its one-hundredth anniversary. In 1922, a group of 39 physicians came together to form what is now known as the American Society for Clinical Pathology (ASCP), now the largest professional organization of pathologists and laboratory professionals in the world.[7] The goal of the ASCP was to “achieve greater scientific proficiency in clinical pathology [meaning here the practice of pathology in the clinical setting, including both anatomical and clinical pathology], and to maintain the status of clinical pathologists on an equal plane with other specialists.”[7] ASCP recognized the contributions and importance of both quality people and quality processes to assuring reliable laboratory results. As such, ASCP was instrumental in creating the American Board of Pathology (1935) to certify pathologists and the Board of Registry (now the Board of Certification) to certify laboratory professionals (1928).[7] ASCP, under the guidance of past president F. William Sunderman, created an inter-laboratory quality control program, now recognized as the College of American Pathologists’ Proficiency Testing Program.[8][9]
The evolution of quality in pathology and laboratory medicine paralleled that of medicine generally. In 1910, Abraham Flexner issued his landmark report that fundamentally restructured medical education in the U.S., a structure still used today. Training moved from a relatively unstructured generalist model to focus on scientific methods with considerably greater rigor.[10] All medical disciplines reexamined their roles in medical education, delivery of healthcare, and patient centricity; pathology and laboratory medicine was no exception.[11][12][13]
In addition to increased technical rigor, attention grew over the ensuing decades to focus on greater evaluation and understanding of the appropriateness (to assess overuse) and necessity (to assess underuse) of medical services. A series of landmark interdisciplinary studies conducted at the RAND Corporation in the 1980s and 1990s highlighted clinical variation in practice and advocated for more explicit criteria to guide diagnosis and treatment.[14][15][16][17] These and other studies served as the basis for more rigorous assessment of clinical evidence, evaluation of practice against that evidence, examination of individual care practices against recognized best practices, and ultimately structuring of payment models to align reimbursement incentives with appropriateness.[18]
Over the past four decades, the discipline of medical quality assessment and improvement has evolved, both scientifically and culturally. Quality control became quality assessment and improvement, and quality improvement became performance improvement. What we recognized originally as performance improvement took a major shift at the turn of the century when the Institute of Medicine published their landmark book To Err Is Human: Building a Safer Health System.[19] This publication and the subsequent publication, Crossing the Quality Chasm: A New Health System for the 21st Century, refocused the dialogue from quality to patient safety.[20] The study and improvement of quality has seen many names over the years; recognizing the goal as patient safety focused attention on what matters most to patients, their safety when seeking care from healthcare systems that exist to reduce the burden of disease and improve outcomes of care.[21]
Patient safety continues to be a primary focus both within healthcare institutions and clinical laboratories. Five decades ago, the laboratory medicine community recognized that laboratory quality depended not only on the analytic quality of the laboratory, but also on the steps that lead up to testing and those that follow. Laboratory quality experts, including the Centers for Disease Control and Prevention, coined the phrase “Total Testing Process,” which encompasses all aspects of testing from the pre-analytical phase starting with formulation of the clinical question that a laboratory test seeks to answer through to the post-analytical phase where the result is reported, acted upon, and impacts patient outcomes.[22][23][24] Studies suggest that the majority (65%–70%) of laboratory errors occur in the preanalytical phase, before the specimen ever reaches the laboratory. Another 15%–20% occur in the postanalytical phase. The fewest errors actually occur in the analytic phase of the total testing process, that part of the total testing process most under the laboratory's direct control.[25]
Emergence of laboratory stewardship
Laboratory stewardship programs focus primarily on aspects of laboratory practice that offer the greatest opportunity to improve patient care, particularly including the pre- and post-analytical components of the total testing process.[26] As such, they now receive increased attention in the overall approach to optimize laboratory services. Stewardship programs challenge laboratory professionals to reach outside their analytic “comfort zone,” recognizing that the greatest opportunity to impact the value of laboratory testing comes from engaging external colleagues to focus on pre- and post-analytic components.[27] Accordingly, laboratorians are strongly encouraged to further develop their existing relationships with clinical partners by engaging in conversations on what constitutes optimal patient care for achieving desired clinical outcomes and reduced diagnostic errors.[6]
The holistic approach of laboratory stewardship to enhance laboratory value aligns with broad healthcare aims. Over the past few decades, healthcare leaders articulated and refined a “triple aim” that includes the key goals of better patient experience, better population health, and lower cost.[28] An additional aim, the well-being of the care team, was added later.[29] Laboratory stewardship supports each of these goals: optimal laboratory testing that avoids over- or undertesting improves the patient experience; reduced care variation improves population health; prevention of unnecessary test orders (and associated downstream care) lowers healthcare costs; and streamlined clinical decision support improves clinician practice satisfaction. In addition to these healthcare goals, laboratory stewardship also acknowledges the operational aspects of laboratory medicine, including risk management and reasonable payment from third party payers for medically necessary services.[30]
Opportunities to improve laboratory services exist in every organization. Some of these opportunities may be unique to a specific organization, while many are common across multiple healthcare systems. When challenges to quality patient care are common, specialty societies, payers, consortia of health systems, and others will articulate those issues and promulgate guidelines, recommendations, payment policies, and other programs to close these common gaps. When developed by these healthcare stakeholders, strategies for optimal care are usually specific, explicit, and based on published evidence. Published guidelines and recommendations from trusted organizations are a good starting place for examination of internal practices because they are defensible, recognized by internal stakeholders, and frequently already tied to performance incentives. Many measures are either laboratory medicine–specific or include laboratory diagnostics metrics to measure performance (e.g., percent of patients with diabetes who are regularly evaluated for glucose control).
The Healthcare Effectiveness Data and Information Set (HEDIS) and the Choosing Wisely campaign are examples of integrated efforts to identify measures that improve quality of patient care, including laboratory services. HEDIS began in the 1990s and is now one of the most widely adopted tools for improving organizational performance. This set of metrics is developed by the National Committee for Quality Assurance (NCQA) and is used by health plans and medical groups to evaluate clinician performance against accepted metrics.[31] Provider incentives are frequently tied to performance on accepted HEDIS measures.
The Choosing Wisely campaign was initiated by the American Board of Internal Medicine (ABIM) Foundation in 2012 to promote conversations among physicians, patients, and other healthcare stakeholders on appropriate patient care.[32] In 2013, the ASCP joined Choosing Wisely to start conversation on the appropriate utilization of medical tests and procedures that sometimes provide little or no benefit and, in some cases, cause harm to patients. A multiyear initiative, the Choosing Wisely campaign provides resources for patients and physicians to engage in these important conversations that help patients choose care that is supported by evidence, not duplicative of other tests and procedures already received, free from harm, and truly appropriate. Choosing Wisely pulls together recommendations from many specialty societies based on published, peer-reviewed evidence. These recommendations, in addition to those published separately by medical specialty societies, are good sources to use when identifying or justifying specific performance improvement projects supported by laboratory stewardship initiatives.
National medical societies such as the ASCP that joined the Choosing Wisely campaign initially identified five tests or procedures often used in their specialty whose use, at least in some situations, should be reconsidered. Numerous Choosing Wisely guidelines that focus on laboratory medicine topics now exist, being submitted by laboratory societies (e.g., ASCP, the American Association of Blood Banks, the American Society for Clinical Laboratory Science, the American Society for Microbiology) and by specialty societies that use laboratory tests (e.g., American College of Obstetricians and Gynecologists, American Academy of Family Physicians).[33][34] For example, in August 2019 the American Academy of Pediatrics recommended not testing for Lyme disease as a cause of musculoskeletal symptoms without an exposure history or appropriate exam findings. They noted: “The musculoskeletal manifestations of Lyme disease include brief attacks of arthralgia with early disseminated Lyme and/or intermittent or persistent episodes of arthritis in one or a few large joints, with predilection for the knee, in late disease. Lyme testing in the absence of these features and without appropriate exposure from living in or traveling to a Lyme endemic area increases the likelihood of false positive results and may lead to unnecessary follow-up and therapy. Diffuse arthralgias, myalgias, or fibromyalgia alone are not criteria for musculoskeletal Lyme disease.”[35]
Implementation of a laboratory stewardship program
Successfully implementing a laboratory stewardship program requires five key elements: a clear vision and organizational alignment, skills, resources, incentives, and a plan of action. Each element, with examples of implementation, is described in detail below.
A clear vision and organizational alignment
Implementing a laboratory stewardship program is an opportunity for laboratory professionals to drive the change they want to see. It is important, however, that the laboratory's vision (i.e., aspirations) is aligned with that of the organization. Absent a clear vision and organizational support, there will be confusion with little chance for meaningful success. Successful program implementation may require a change or alignment of culture (i.e., the values, beliefs, and norms influencing how people behave as members of an organization) and a shared understanding of why change is needed.[36] Critical to this change management is a solid laboratory professional–clinician partnership.
Understand the health system organization and establish key partnerships with stakeholders
When initiating a laboratory stewardship program, one must first understand the health system's organization and culture. Laboratory stewardship is by nature interdisciplinary, and understanding the health system enables identification of key stakeholders across the organization with whom laboratory professionals will need to partner to achieve desired clinical outcomes. Key stakeholders often include health system medical (e.g., physicians, nurses, physician assistants, pharmacists) and administrative (e.g., safety managers, information technology analysts, quality improvement specialists, members of the “C-suite”) leadership in addition to laboratory medical and administrative leadership.
Traditional organizational structures are changing in response to pressures throughout healthcare; now, organizational structures vary such that an understanding of one does not likely extend to an understanding of others. Traditional departmental medical staff structures still maintain important roles, but alternative structures such as service or product lines have developed to provide an interdisciplinary, integrated care approach when managing specific patient populations (e.g., cardiology, oncology). Therefore, understand how physicians, other healthcare providers, and administrators interact to achieve desired system outcomes so that the stewardship initiative can be integrated smoothly. For example, leaders and departments tasked with improving budget and financial scorecards may have specific targets; laboratory initiatives can help them reach their targets. Health systems usually have working committees focused on quality, infection control, medication management, and medical staff privileging. An existing committee may already be tasked with elements of laboratory stewardship, in which case the stewardship initiative should be integrated carefully and with buy-in from these stakeholders.
Given the importance of physician leadership to every laboratory stewardship program, time is needed to understand the medical staff who use laboratory services. Some physicians may be employed; others might be independent or contracted. These relationships may become relevant when selecting program participants. Alignment may be more difficult when physicians are independent because if their income is generated solely through direct patient care, investing many hours on an “extracurricular” endeavor results in lost income, unless the system compensates individuals for time lost from direct patient care.
Establish leadership and governance for the laboratory stewardship program
A laboratory stewardship program requires dedicated oversight to focus on identifying and implementing needed changes. Before establishing the program's oversight structure, however, recognize how the key administrative (e.g., CEO/COO for the system, or the chief technologist in the laboratory) and medical (e.g., CMO/director of medical affairs for the system, or laboratory director/CLIA certificate holder in the laboratory) leaders intersect in terms of their roles and responsibilities. This is important both to make sure the right people are engaged as initiatives are undertaken and to assure that organizational structures and lines of authority are respected.
The oversight structure may be a specific committee, or a subcommittee or task force, of an existing health system committee (Fig. 1). Participants in stewardship oversight should include stakeholders from the laboratory, inpatient and ambulatory providers, and administrative leaders.[26] Laboratory participants may include pathologists, operational directors, and medical laboratory scientists. Clinical participants may include primary care and specialist clinicians and other staff (e.g., nurse practitioners, nurses, and physician assistants). Health system administrators (e.g., finance, information technology, risk management and process improvement) are important to help integrate and align the program with the broader organization. Additional individuals may participate based on their subject matter expertise related to specific program initiatives.
|
Successful programs have a specific point of accountability (e.g., a designated leader or two co-leaders). There must also be a knowledgeable staff person (e.g., a program manager) specifically assigned to the laboratory stewardship initiative. The staff person must keep the program on track by aggregating data, coordinating analyst and other team member activities, scheduling meetings, engaging subject matter experts, and ensuring the leader or co-leaders are always apprised of activities.
Communicate a compelling case for change
Stakeholders must be convinced of the need for a laboratory stewardship program. Laboratory-based stewardship champions must create enthusiasm to secure non-laboratory stakeholder buy-in to create the true interdisciplinary team. Once non-laboratory stakeholders support the program, they can also become stewardship champions who help others recognize the value of laboratory services and secure additional buy-in for the program. The interdisciplinary approach helps assure initial efforts are integrated, make operational sense, and progress toward achieving desired clinical outcomes.
Laboratory professionals can actively cultivate other stakeholders to become stewardship champions. Laboratory medical and technologist members who serve on organizational committees may already have established relationships and credibility with patient-facing physicians and clinical staff. Look to existing successful laboratory-hospital partnerships such as effective blood utilization and antimicrobial stewardship initiatives. Laboratory stewardship can thus be framed as an extension of existing partnerships.
Compelling case discussions should include presentation of data (e.g., declining performance on HEDIS indicators) to establish the importance of selected initiatives and provide the basis for change, followed by discussions on desired outcomes and how to achieve them. Peer-to-peer conversations, rather than lectures that come across as patronizing, can effectively educate stakeholders and promote alignment. When aligning on desired outcomes, the focus should be on achieving beneficial patient outcomes and not simply achieving appropriate laboratory utilization (e.g., correct test ordering patterns). This framing is more inclusive, should align with the organization's strategic plan, and highlights a shared value of laboratorians and clinicians.
Discussions allow stakeholders to share diverse perspectives. Ongoing engagement, especially across departments and disciplines, leads to identification of challenges and opportunities that may not otherwise be recognized. Through collaborative discussion, stewardship champions and key stakeholders can begin developing initial project proposals. Initial projects should each have a reasonable scope so that early program initiatives have the best chance to succeed and prove value. Early successes promote further change and should be widely shared across the organization to build program support. The team can then move on to bigger challenges, including looking at changing deeply ingrained practice challenges.
Skills
A laboratory stewardship program requires analytic, subject matter, presentation, project management, interpersonal, and leadership skills. Rarely all the needed skills to carry out a successful laboratory stewardship program will be embodied in one person. That is precisely why an effective team brings multiple colleagues to the table, each with unique skills and abilities, to define, evaluate, and address problems.
Analytic skills
References
- ↑ Forsman, R W (1 May 1996). "Why is the laboratory an afterthought for managed care organizations?" (in en). Clinical Chemistry 42 (5): 813–816. doi:10.1093/clinchem/42.5.813. ISSN 0009-9147. https://academic.oup.com/clinchem/article/42/5/813/5646564.
- ↑ Centers for Disease Control and Prevention (17 March 2021). "Overview of Testing for SARS-CoV-2 (COVID-19)". COVID-19 Portal. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/hcp/testing-overview.html. Retrieved 30 March 2021.
- ↑ World Health Organization (24 February 2021). "Looking back at a year that changed the world: WHO’s response to COVID-19". World Health Organization. https://www.who.int/publications/m/item/looking-back-at-a-year-that-changed-the-world-who-s-response-to-covid-19. Retrieved 30 March 2021.
- ↑ Centers for Medicare & Medicaid Services (3 December 2020). "Physician and Other Supplier Data CY 2018". Centers for Medicare & Medicaid Services. https://www.cms.gov/research-statistics-data-systems/medicare-provider-utilization-and-payment-data/medicare-provider-utilization-and-payment-data-physician-and-other-supplier/physician-and-other-supplier-data-cy-2018. Retrieved 30 March 2021.
- ↑ Shrank, William H.; Rogstad, Teresa L.; Parekh, Natasha (15 October 2019). "Waste in the US Health Care System: Estimated Costs and Potential for Savings" (in en). JAMA 322 (15): 1501. doi:10.1001/jama.2019.13978. ISSN 0098-7484. https://jamanetwork.com/journals/jama/fullarticle/2752664.
- ↑ 6.0 6.1 Committee on Diagnostic Error in Health Care; Board on Health Care Services; Institute of Medicine; The National Academies of Sciences, Engineering, and Medicine (29 December 2015). Balogh, Erin P.; Miller, Bryan T.; Ball, John R.. eds. Improving Diagnosis in Health Care. Washington, D.C.: National Academies Press. doi:10.17226/21794. ISBN 978-0-309-37769-0. http://www.nap.edu/catalog/21794.
- ↑ 7.0 7.1 7.2 Rodriguez, Fred H.; Ball, John R. (1 October 2007). "The American Society for Clinical Pathology: The Pathology Society of “Firsts”" (in en). Laboratory Medicine 38 (10): 595–601. doi:10.1309/RTKE4EKQ61HMN37U. ISSN 0007-5027. https://academic.oup.com/labmed/article-lookup/doi/10.1309/RTKE4EKQ61HMN37U.
- ↑ Lundberg, G.D. (27 June 2003). "The Pathologist of the Century -- F. William Sunderman, MD, PhD, ScD". Medscape. WebMD, LLC. https://www.medscape.com/viewarticle/457676. Retrieved 05 April 2021.
- ↑ Belk, William P.; Sunderman, F. William (1 November 1947). "A Survey of the Accuracy of Chemical Analyses in Clinical Laboratories" (in en). American Journal of Clinical Pathology 17 (11): 853–861. doi:10.1093/ajcp/17.11.853. ISSN 0002-9173. https://academic.oup.com/ajcp/article-lookup/doi/10.1093/ajcp/17.11.853.
- ↑ Flexner, A. (1910). "Medical Education in the United States and Canada: A Report to the Carnegie Foundation for the Advancement of Teaching" (PDF). Carnegie Foundation for the Advancement of Teaching. http://archive.carnegiefoundation.org/publications/pdfs/elibrary/Carnegie_Flexner_Report.pdf. Retrieved 30 March 2021.
- ↑ Andresen, Marjory I.; Mugrage, Edward R. (1 January 1938). "Venous and Peripheral Red Blood Cell Values" (in en). American Journal of Clinical Pathology 8 (1): 46–51. doi:10.1093/ajcp/8.1.46. ISSN 0002-9173. https://academic.oup.com/ajcp/article-lookup/doi/10.1093/ajcp/8.1.46.
- ↑ Curphey, Theodore J. (1 January 1949). "Widening Horizons in Pathology" (in en). American Journal of Clinical Pathology 19 (1): 1–9. doi:10.1093/ajcp/19.1.1. ISSN 0002-9173. https://academic.oup.com/ajcp/article-lookup/doi/10.1093/ajcp/19.1.1.
- ↑ Savignano, Thomas; Hanok, Albert; Kuo, Jeremiah (1 January 1969). "Creatine Phosphokinase Activity: A Study of Normal and Abnormal Levels" (in en). American Journal of Clinical Pathology 51 (1): 76–85. doi:10.1093/ajcp/51.1.76. ISSN 0002-9173. https://academic.oup.com/ajcp/article-lookup/doi/10.1093/ajcp/51.1.76.
- ↑ Park, R E; Fink, A; Brook, R H; Chassin, M R; Kahn, K L; Merrick, N J; Kosecoff, J; Solomon, D H (1 July 1986). "Physician ratings of appropriate indications for six medical and surgical procedures." (in en). American Journal of Public Health 76 (7): 766–772. doi:10.2105/AJPH.76.7.766. ISSN 0090-0036. PMC PMC1646864. PMID 3521341. http://ajph.aphapublications.org/doi/10.2105/AJPH.76.7.766.
- ↑ McClellan, Mark; Brook, Robert H. (1 July 1992). "Appropriateness of Care: A Comparison of Global and Outcome Methods to Set Standards" (in en). Medical Care 30 (7): 565–586. doi:10.1097/00005650-199207000-00001. ISSN 0025-7079. http://journals.lww.com/00005650-199207000-00001.
- ↑ Kahan, James P.; Bernstein, Steven J.; Leape, Lucian L.; Hilborne, Lee H.; Park, Rolla Edward; Parker, Lori; Kamberg, Caren J.; Brook, Robert H. (1 April 1994). "Measuring the Necessity of Medical Procedures:" (in en). Medical Care 32 (4): 357–365. doi:10.1097/00005650-199404000-00004. ISSN 0025-7079. http://journals.lww.com/00005650-199404000-00004.
- ↑ Leape, Lucian L. (2 February 1999). "Underuse of Cardiac Procedures: Do Women, Ethnic Minorities, and the Uninsured Fail To Receive Needed Revascularization?" (in en). Annals of Internal Medicine 130 (3): 183. doi:10.7326/0003-4819-130-3-199902020-00003. ISSN 0003-4819. http://annals.org/article.aspx?doi=10.7326/0003-4819-130-3-199902020-00003.
- ↑ Kosecoff, Jacqueline (17 October 1990). "Prospective Payment System and Impairment at Discharge: The `Quicker-and-Sicker' Story Revisited" (in en). JAMA 264 (15): 1980. doi:10.1001/jama.1990.03450150080035. ISSN 0098-7484. http://jama.jamanetwork.com/article.aspx?doi=10.1001/jama.1990.03450150080035.
- ↑ Institute of Medicine (1 March 2000). To Err Is Human: Building a Safer Health System. Washington, D.C.: National Academies Press. doi:10.17226/9728. ISBN 978-0-309-26174-6. http://www.nap.edu/catalog/9728.
- ↑ Institute of Medicine (19 July 2001). Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, D.C.: National Academies Press. doi:10.17226/10027. ISBN 978-0-309-07280-9. http://www.nap.edu/catalog/10027.
- ↑ Youngberg, Barbara J.; Hatlie, Martin J., eds. (2004). The patient safety handbook. Sudbury, MA: Jones and Bartlett. ISBN 978-0-7637-3147-2.
- ↑ Martin, M.L.; Addison, B.V.; Wagner, W.M. et al., ed. (1991). Proceedings of the 1989 Institute on Critical Issues in Health Laboratory Practice Improving the Quality of Health management Through Clinician and Laboratorian Teamwork (The Du Pont Co).
- ↑ Schumacher, Gerald E; Barr, Judith T (1 February 1998). "Total testing process applied to therapeutic drug monitoring: impact on patients’ outcomes and economics" (in en). Clinical Chemistry 44 (2): 370–374. doi:10.1093/clinchem/44.2.370. ISSN 0009-9147. https://academic.oup.com/clinchem/article/44/2/370/5642676.
- ↑ Plebani, Mario; Laposata, Michael; Lundberg, George D. (1 December 2011). "The Brain-to-Brain Loop Concept for Laboratory Testing 40 Years After Its Introduction" (in en). American Journal of Clinical Pathology 136 (6): 829–833. doi:10.1309/AJCPR28HWHSSDNON. ISSN 1943-7722. https://academic.oup.com/ajcp/article/136/6/829/1760426.
- ↑ Plebani, Mario; Carraro, Paolo (1 August 1997). "Mistakes in a stat laboratory: types and frequency" (in en). Clinical Chemistry 43 (8): 1348–1351. doi:10.1093/clinchem/43.8.1348. ISSN 0009-9147. https://academic.oup.com/clinchem/article/43/8/1348/5641004.
- ↑ 26.0 26.1 Dickerson, Jane A.; Fletcher, Andrew H.; Procop, Gary; Keren, David F.; Singh, Ila R.; Garcia, Joaquin J.; Carpenter, Robert B.; Miles, Joe et al. (1 September 2017). "Transforming Laboratory Utilization Review into Laboratory Stewardship: Guidelines by the PLUGS National Committee for Laboratory Stewardship" (in en). The Journal of Applied Laboratory Medicine: An AACC Publication 2 (2): 259–268. doi:10.1373/jalm.2017.023606. ISSN 2475-7241. https://academic.oup.com/jalm/article/2/2/259-268/5587542.
- ↑ Baird, Geoffrey S. (26 March 2019). "The Choosing Wisely initiative and laboratory test stewardship" (in en). Diagnosis 6 (1): 15–23. doi:10.1515/dx-2018-0045. ISSN 2194-802X. https://www.degruyter.com/document/doi/10.1515/dx-2018-0045/html.
- ↑ Berwick, Donald M.; Nolan, Thomas W.; Whittington, John (1 May 2008). "The Triple Aim: Care, Health, And Cost" (in en). Health Affairs 27 (3): 759–769. doi:10.1377/hlthaff.27.3.759. ISSN 0278-2715. http://www.healthaffairs.org/doi/10.1377/hlthaff.27.3.759.
- ↑ Bodenheimer, T.; Sinsky, C. (1 November 2014). "From Triple to Quadruple Aim: Care of the Patient Requires Care of the Provider" (in en). The Annals of Family Medicine 12 (6): 573–576. doi:10.1370/afm.1713. ISSN 1544-1709. PMC PMC4226781. PMID 25384822. http://www.annfammed.org/cgi/doi/10.1370/afm.1713.
- ↑ Astion, M.; Dickerson, J. (1 January 2019). "Laboratory Stewardship Focus: A New Quarterly Section in Clinical Laboratory News". Clinical Laboratory News. American Association of Clinical Chemistry. https://www.aacc.org/cln/articles/2019/janfeb/laboratory-stewardship-focus-a-new-quarterly-section-in-clinical-laboratory-news. Retrieved 30 March 2021.
- ↑ National Committee for Quality Assurance. "HEDIS Measures and Technical Resources". National Committee for Quality Assurance. https://www.ncqa.org/hedis/measures/. Retrieved 30 March 2021.
- ↑ Hilborne, L.H. (18 May 2014). "Choosing Wisely: Selecting the right test for the right patient at the right time". Medical Laboratory Observer. https://www.mlo-online.com/home/article/13006611/choosing-wisely-selecting-the-right-test-for-the-right-patient-at-the-right-time.
- ↑ ABIM Foundation. "Clinician Lists". Choosing Wisely. ABIM Foundation. https://www.choosingwisely.org/clinician-lists/. Retrieved 30 March 2021.
- ↑ Waibel, Elizabeth; Garcia, Edna; Kelly, Melissa; Soles, Ryan; Hilborne, Lee (17 February 2018). "Systematic Review of Non-ASCP Choosing Wisely Recommendations Relevant to Pathology and Laboratory Medicine" (in en). American Journal of Clinical Pathology 149 (3): 267–274. doi:10.1093/ajcp/aqx159. ISSN 0002-9173. https://academic.oup.com/ajcp/article/149/3/267/4841631.
- ↑ American Academy of Pediatrics (6 August 2019). "Do not test for Lyme disease as a cause of musculoskeletal symptoms without an exposure history or appropriate exam findings.". Choosing Wisely. ABIM Foundation. https://www.choosingwisely.org/clinician-lists/aap-sorh-testing-for-lyme-disease-with-musculoskeletal-symptoms/. Retrieved 30 March 2021.
- ↑ Ravasi, Davide; Schultz, Majken (1 June 2006). "Responding to Organizational Identity Threats: Exploring the Role of Organizational Culture" (in en). Academy of Management Journal 49 (3): 433–458. doi:10.5465/amj.2006.21794663. ISSN 0001-4273. http://journals.aom.org/doi/10.5465/amj.2006.21794663.
Notes
This presentation is faithful to the original, with only a few minor changes to presentation, grammar, and punctuation. In some cases important information was missing from the references, and that information was added.