Difference between revisions of "Main Page/Featured article of the week/2016"
Shawndouglas (talk | contribs) (Added last week's article of the week.) |
Shawndouglas (talk | contribs) (Added last week's article of the week.) |
||
| Line 14: | Line 14: | ||
{| id="mp-right" style="width:100%; vertical-align:top; background:#f5faff;" | {| id="mp-right" style="width:100%; vertical-align:top; background:#f5faff;" | ||
|- | |- | ||
|- | |||
<!-- Below this line begin pasting previous news --> | |||
<h2 style="font-size:105%; font-weight:bold; text-align:left; color:#000; padding:0.2em 0.4em; width:50%;">Featured article of the week: January 11–17:</h2> | |||
<div style="padding:0.4em 1em 0.3em 1em;"> | |||
<div style="float: left; margin: 0.5em 0.9em 0.4em 0em;">[[File:Fig1 Calabria BMCBioinformatics2015 16-Suppl9.jpg|220px]]</div> | |||
'''"[[Journal:adLIMS: A customized open source software that allows bridging clinical and basic molecular research studies|adLIMS: A customized open source software that allows bridging clinical and basic molecular research studies]]"''' | |||
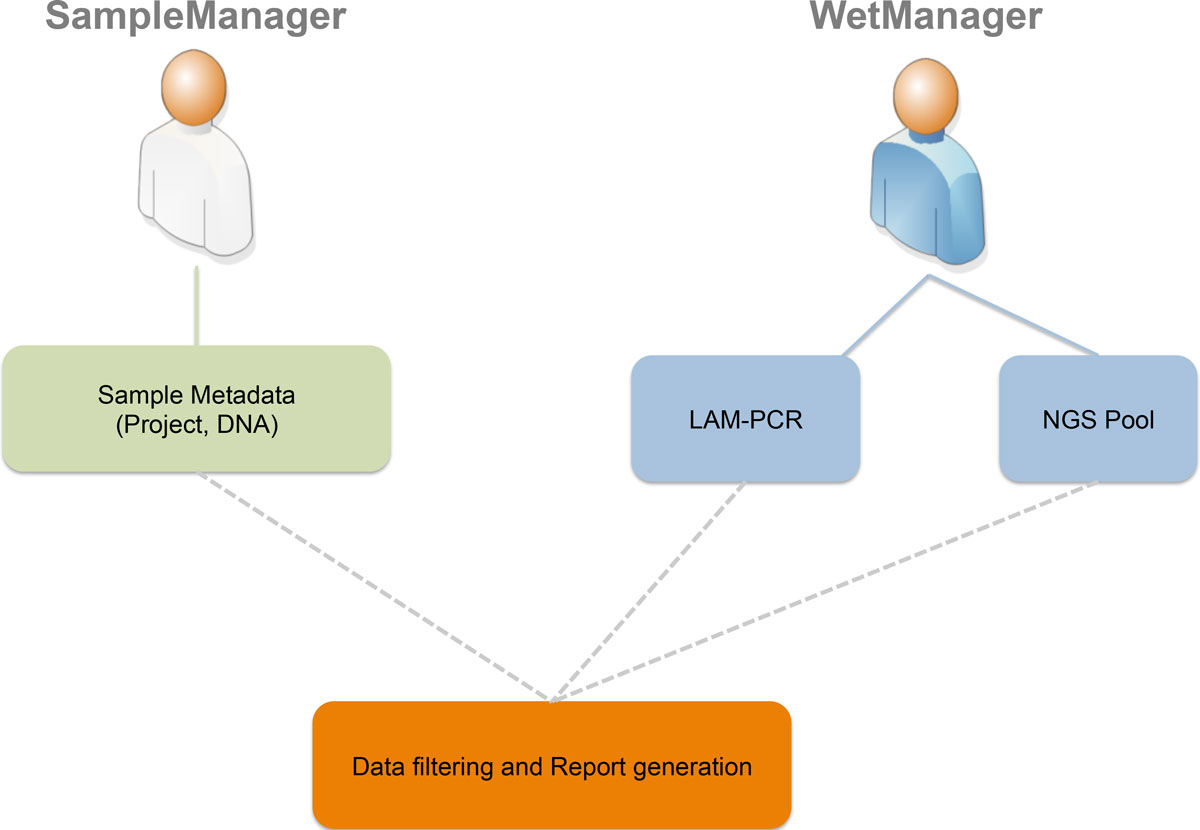
Many biological laboratories that deal with genomic samples are facing the problem of sample tracking, both for pure [[laboratory]] management and for efficiency. Our laboratory exploits PCR techniques and Next Generation Sequencing (NGS) methods to perform high-throughput integration site monitoring in different clinical trials and scientific projects. Because of the huge amount of samples that we process every year, which result in hundreds of millions of sequencing reads, we need to standardize data management and tracking systems, building up a scalable and flexible structure with web-based interfaces, which are usually called [[Laboratory information management system|Laboratory Information Management System]] (LIMS). | |||
We extended and customized [[ADempiere]] ERP to fulfill LIMS requirements and we developed adLIMS. It has been validated by our end-users verifying functionalities and GUIs through test cases for PCRs samples and pre-sequencing data and it is currently in use in our laboratories. adLIMS implements authorization and authentication policies, allowing multiple users management and roles definition that enables specific permissions, operations and data views to each user. ('''[[Journal:adLIMS: A customized open source software that allows bridging clinical and basic molecular research studies|Full article...]]''')<br /> | |||
</div> | |||
|- | |- | ||
|<br /><h2 style="font-size:105%; font-weight:bold; text-align:left; color:#000; padding:0.2em 0.4em; width:50%;">Featured article of the week: January 4–10:</h2> | |<br /><h2 style="font-size:105%; font-weight:bold; text-align:left; color:#000; padding:0.2em 0.4em; width:50%;">Featured article of the week: January 4–10:</h2> | ||
| Line 25: | Line 37: | ||
</div> | </div> | ||
|- | |- | ||
|} | |} | ||
|} | |} | ||
<br /> | <br /> | ||
<div align="center">[[Main Page|— Return to the front page —]]</div> | <div align="center">[[Main Page|— Return to the front page —]]</div> | ||
Revision as of 16:04, 19 January 2016
|
|
If you're looking for the 2014 archive, it can be found here. The 2015 archive is here. |
Featured article of the week archive - 2016
Welcome to the LIMSwiki 2016 archive for the Featured Article of the Week.
Featured article of the week: January 11–17:Many biological laboratories that deal with genomic samples are facing the problem of sample tracking, both for pure laboratory management and for efficiency. Our laboratory exploits PCR techniques and Next Generation Sequencing (NGS) methods to perform high-throughput integration site monitoring in different clinical trials and scientific projects. Because of the huge amount of samples that we process every year, which result in hundreds of millions of sequencing reads, we need to standardize data management and tracking systems, building up a scalable and flexible structure with web-based interfaces, which are usually called Laboratory Information Management System (LIMS). We extended and customized ADempiere ERP to fulfill LIMS requirements and we developed adLIMS. It has been validated by our end-users verifying functionalities and GUIs through test cases for PCRs samples and pre-sequencing data and it is currently in use in our laboratories. adLIMS implements authorization and authentication policies, allowing multiple users management and roles definition that enables specific permissions, operations and data views to each user. (Full article...)
|