File:Fig4 Smith PracLabMed2024 InPress.jpg
Original file (2,764 × 1,217 pixels, file size: 178 KB, MIME type: image/jpeg)
Summary
| Description |
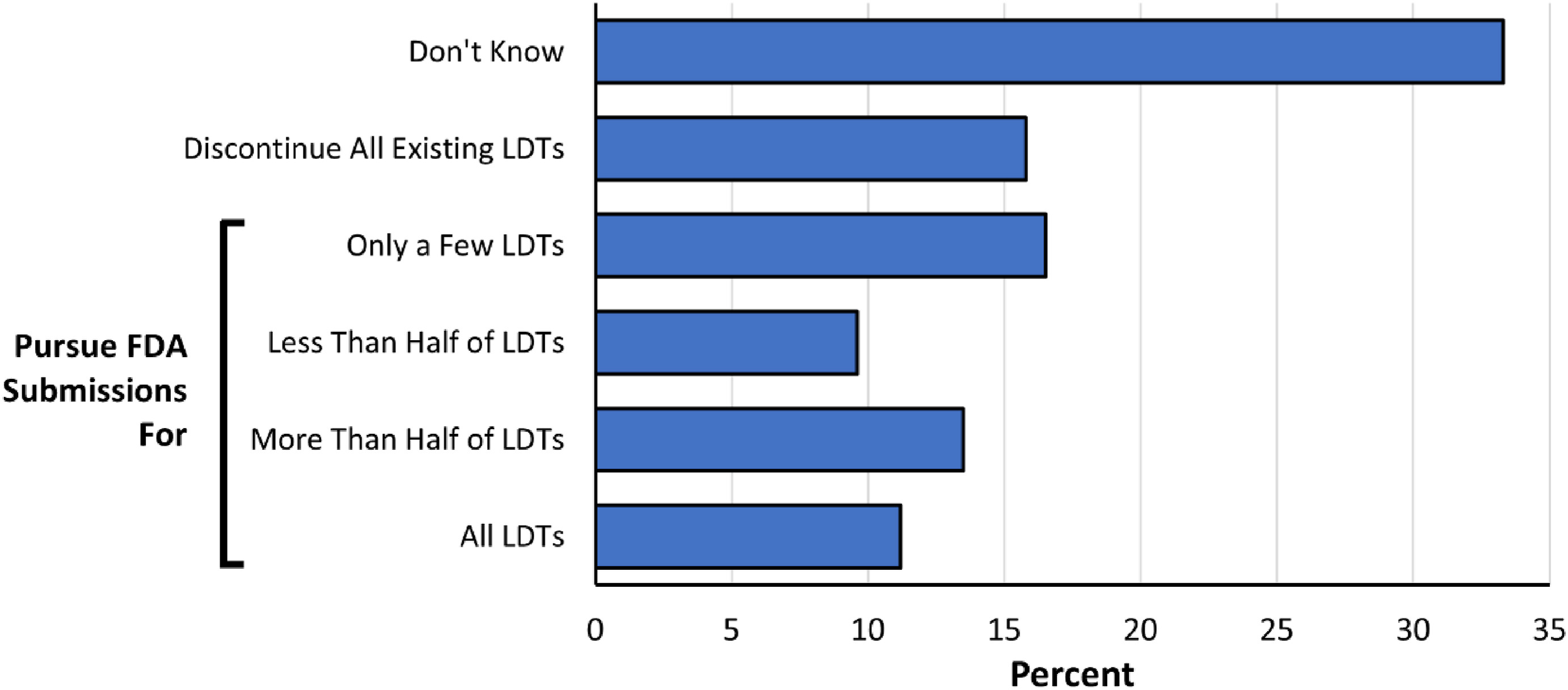
Figure 4. Anticipated response to the proposed rule. Respondents were asked how they think their laboratories would respond to the new regulatory requirements regarding their existing LDTs if the FDA adopts the proposed rule. |
|---|---|
| Source |
Smith, Leslie; Carricaburu, Lisa A.; Genzen, Jonathan R. (2024). "The US FDA’s proposed rule on laboratory-developed tests: Impacts on clinical laboratory testing". Practical Laboratory Medicine In press: e00407. doi:10.1016/j.plabm.2024.e00407. |
| Date |
2024 |
| Author |
Smith, Leslie; Carricaburu, Lisa A.; Genzen, Jonathan R. |
| Permission (Reusing this file) |
Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International |
| Other versions |
Licensing
|
|
This work is licensed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License. |
File history
Click on a date/time to view the file as it appeared at that time.
| Date/Time | Thumbnail | Dimensions | User | Comment | |
|---|---|---|---|---|---|
| current | 20:14, 26 May 2024 |  | 2,764 × 1,217 (178 KB) | Shawndouglas (talk | contribs) |
You cannot overwrite this file.
File usage
The following page uses this file:









