Difference between revisions of "Template:Article of the week"
Shawndouglas (talk | contribs) (Updated article of the week text) |
Shawndouglas (talk | contribs) (Updated article of the week text) |
||
| Line 1: | Line 1: | ||
<div style="float: left; margin: 0.5em 0.9em 0.4em 0em;">[[File:Fig1 | <div style="float: left; margin: 0.5em 0.9em 0.4em 0em;">[[File:Fig1 Gonzales PLOSComBio22 18-8.png|240px]]</div> | ||
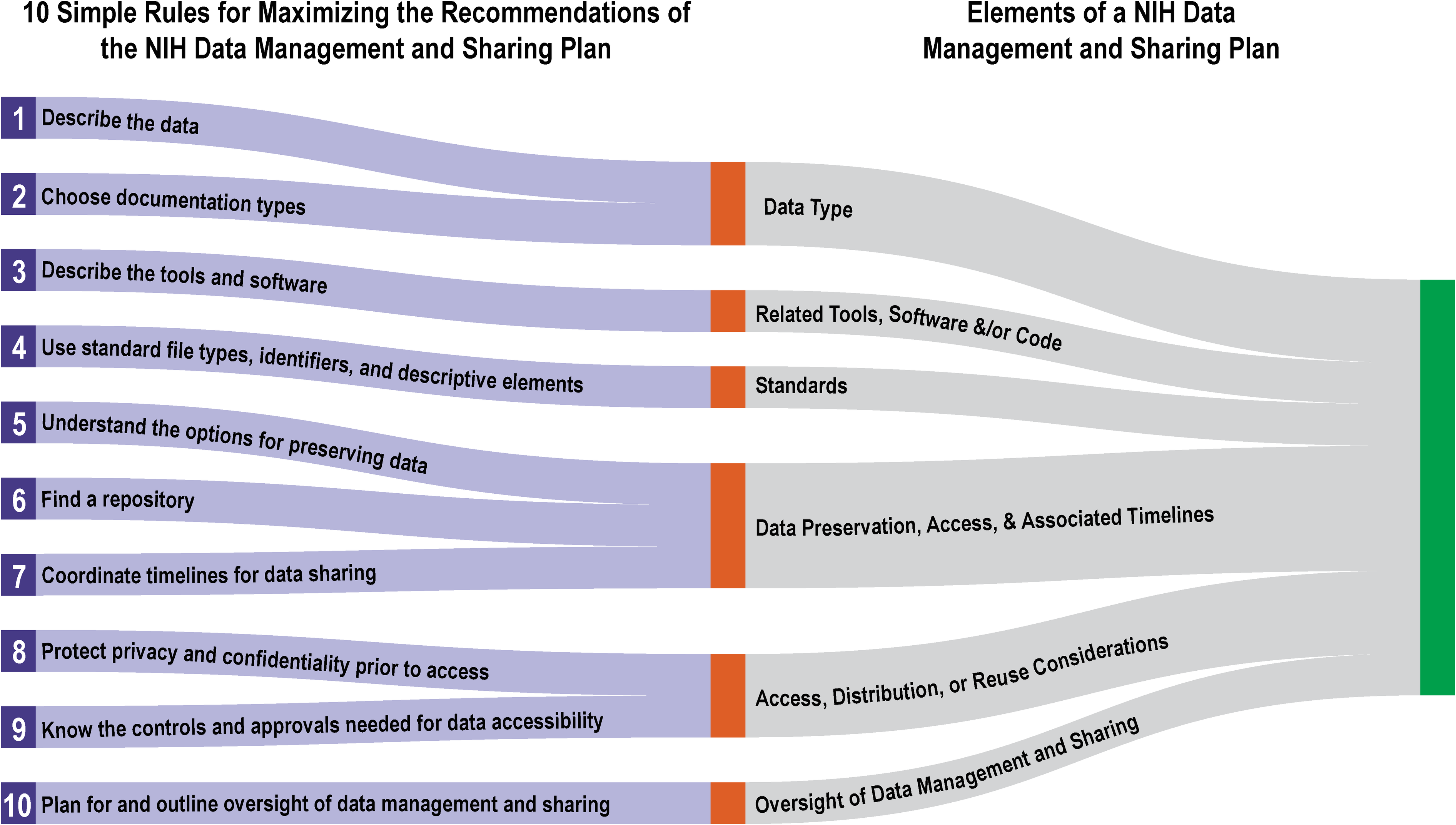
'''"[[Journal: | '''"[[Journal:Ten simple rules for maximizing the recommendations of the NIH data management and sharing plan|Ten simple rules for maximizing the recommendations of the NIH data management and sharing plan]]"''' | ||
The [[National Institutes of Health]] (NIH) Policy for Data Management and Sharing (DMS Policy) recognizes the NIH’s role as a key steward of the United States' biomedical research and information and seeks to enhance that stewardship through systematic recommendations for the preservation and [[Data sharing|sharing]] of research data generated by funded projects. The policy is effective as of January 2023. The recommendations include a requirement for the submission of a data management and sharing plan (DMSP) with funding applications, and while no strict template was provided, the NIH has released supplemental draft guidance on elements to consider when developing such a plan. This article provides 10 key recommendations for creating a DMSP that is both maximally compliant and effective. ('''[[Journal:Ten simple rules for maximizing the recommendations of the NIH data management and sharing planFull article...]]''')<br /> | |||
''Recently featured'': | ''Recently featured'': | ||
{{flowlist | | {{flowlist | | ||
* [[Journal:Understanding cybersecurity frameworks and information security standards: A review and comprehensive overview|Understanding cybersecurity frameworks and information security standards: A review and comprehensive overview]] | |||
* [[Journal:Bridging data management platforms and visualization tools to enable ad-hoc and smart analytics in life sciences|Bridging data management platforms and visualization tools to enable ad-hoc and smart analytics in life sciences]] | * [[Journal:Bridging data management platforms and visualization tools to enable ad-hoc and smart analytics in life sciences|Bridging data management platforms and visualization tools to enable ad-hoc and smart analytics in life sciences]] | ||
* [[Journal:Digitalization of calibration data management in the pharmaceutical industry using a multitenant platform|Digitalization of calibration data management in the pharmaceutical industry using a multitenant platform]] | * [[Journal:Digitalization of calibration data management in the pharmaceutical industry using a multitenant platform|Digitalization of calibration data management in the pharmaceutical industry using a multitenant platform]] | ||
}} | }} | ||
Revision as of 16:47, 31 July 2023
"Ten simple rules for maximizing the recommendations of the NIH data management and sharing plan"
The National Institutes of Health (NIH) Policy for Data Management and Sharing (DMS Policy) recognizes the NIH’s role as a key steward of the United States' biomedical research and information and seeks to enhance that stewardship through systematic recommendations for the preservation and sharing of research data generated by funded projects. The policy is effective as of January 2023. The recommendations include a requirement for the submission of a data management and sharing plan (DMSP) with funding applications, and while no strict template was provided, the NIH has released supplemental draft guidance on elements to consider when developing such a plan. This article provides 10 key recommendations for creating a DMSP that is both maximally compliant and effective. (Journal:Ten simple rules for maximizing the recommendations of the NIH data management and sharing planFull article...)
Recently featured:
- Understanding cybersecurity frameworks and information security standards: A review and comprehensive overview
- Bridging data management platforms and visualization tools to enable ad-hoc and smart analytics in life sciences
- Digitalization of calibration data management in the pharmaceutical industry using a multitenant platform